Macrophages archive HIV-1 virions for dissemination in trans
- PMID: 15920469
- PMCID: PMC1173148
- DOI: 10.1038/sj.emboj.7600707
Macrophages archive HIV-1 virions for dissemination in trans
Abstract
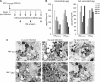
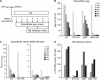
Viruses have evolved various strategies in order to persist within the host. To date, most information on mechanisms of HIV-1 persistence has been derived from studies with lymphocytes, but there is little information regarding mechanisms that govern HIV-1 persistence in macrophages. It has previously been demonstrated that virus assembly in macrophages occurs in cytoplasmic vesicles, which exhibit the characteristics of multivesicular bodies or late endosomes. The infectious stability of virions that assemble intracellularly in macrophages has not been evaluated. We demonstrate that virions assembling intracellularly in primary macrophages retain infectivity for extended intervals. Infectious virus was recovered directly from cytoplasmic lysates of macrophages and could be transmitted from macrophages to peripheral blood lymphocytes in trans 6 weeks after ongoing viral replication was blocked. Cell-associated virus decayed significantly from 1 to 2 weeks post infection, but decreased minimally thereafter. The persistence of intracellular virions did not require the viral accessory proteins Vpu or Nef. The stable sequestration of infectious virions within cytoplasmic compartments of macrophages may represent an additional mechanism for viral persistence in HIV-1-infected individuals.
Figures





References
-
- Biswas P, Poli G, Kinter A, Justement J, Stanley SK, Maury W, Bressler P, Orenstein JM, Fauci AS (1992) Interferon gamma induces the expression of human immunodeficiency virus in persistently infected promonocytic cells (U1) and redirects the production of virions to intracytoplasmic vacuoles in phorbol myristate acetate-differentiated U1 cells. J Exp Med 176: 739–750 - PMC - PubMed
-
- Bour S, Strebel K (2003) The HIV-1 Vpu protein: a mulifunctional enhancer of viral particle release. Microbes Infect 5: 1029–1039 - PubMed
-
- Brichacek B, Stevenson M (1997) Quantitative competitive RNA PCR for quantitation of virion associated HIV-1 RNA. Methods 12: 294–299 - PubMed
-
- Cameron PU, Freudenthal PS, Barker JM, Gezelter S, Inaba I, Steinman RM (1992) Dendritic cells exposed to human immunodeficiency virus type-1 transmit a vigorous cytopathic infection to CD4+ T cells. Science 257: 383–387 - PubMed
-
- Carr JM, Hocking H, Li P, Burrell CJ (1999) Rapid and efficient cell-to-cell transmission of human immunodeficiency virus infection from monocyte-derived macrophages to peripheral blood lymphocytes. Virology 265: 319–329 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

