v-SNAREs control exocytosis of vesicles from priming to fusion
- PMID: 15920476
- PMCID: PMC1150890
- DOI: 10.1038/sj.emboj.7600696
v-SNAREs control exocytosis of vesicles from priming to fusion
Abstract
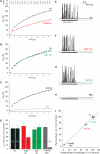
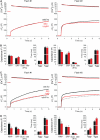
SNARE proteins (soluble NSF-attachment protein receptors) are thought to be central components of the exocytotic mechanism in neurosecretory cells, but their precise function remained unclear. Here, we show that each of the vesicle-associated SNARE proteins (v-SNARE) of a chromaffin granule, synaptobrevin II or cellubrevin, is sufficient to support Ca(2+)-dependent exocytosis and to establish a pool of primed, readily releasable vesicles. In the absence of both proteins, secretion is abolished, without affecting biogenesis or docking of granules indicating that v-SNAREs are absolutely required for granule exocytosis. We find that synaptobrevin II and cellubrevin differentially control the pool of readily releasable vesicles and show that the v-SNARE's amino terminus regulates the vesicle's primed state. We demonstrate that dynamics of fusion pore dilation are regulated by v-SNAREs, indicating their action throughout exocytosis from priming to fusion of vesicles.
Figures





References
-
- Albillos A, Dernick G, Horstmann H, Almers W, Alvarez de Toledo G, Lindau M (1997) The exocytotic event in chromaffin cells revealed by patch amperometry. Nature 389: 509–512 - PubMed
-
- Ashery U, Betz A, Xu T, Brose N, Rettig J (1999) An efficient method for infection of adrenal chromaffin cells using the Semliki Forest virus gene expression system. Eur J Cell Biol 78: 525–532 - PubMed
-
- Bezzi P, Gundersen V, Galbete JL, Seifer G, Steinhauser C, Pilati E, Volterra A (2004) Astrocytes contain a vesicular compartment that is competent for regulated exocytosis of glutamate. Nat Neurosci 7: 613–620 - PubMed
-
- Bhattacharya S, Stewart BA, Niemeyer BA, Burgess RW, McCabe BD, Lin P, Boulianne G, O'Kane CJ, Schwarz TL (2002) Members of the synaptobrevin/vesicle-associated membrane protein (VAMP) family in Drosophila are functionally interchangeable in vivo for neurotransmitter release and cell viability. Proc Natl Acad Sci USA 99: 13867–13872 - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

