Bax forms multispanning monomers that oligomerize to permeabilize membranes during apoptosis
- PMID: 15920484
- PMCID: PMC1150878
- DOI: 10.1038/sj.emboj.7600675
Bax forms multispanning monomers that oligomerize to permeabilize membranes during apoptosis
Abstract
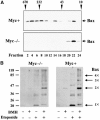
Bax promotes cell death by permeabilizing mitochondrial outer membranes by an unresolved mechanism. However, in cells lacking the gene c-myc, membrane permeabilization by Bax is blocked by changes in the mitochondria that prevent Bax oligomerization. Drug-treated c-myc null cells and cells expressing Myc were used to map the topology of Bax in membranes prior to and after mitochondrial permeabilization. Chemical labeling of single cysteine mutants of Bax using a membrane bilayer impermeant cysteine-specific modifying agent revealed that Bax inserted both the 'pore domain' (helices alpha5-alpha6), and the tail-anchor (helix alpha9) into membranes prior to oligomerization and membrane permeabilization. Additional topology changes for Bax were not required in Myc-expressing cells to promote oligomerization and cytochrome c release. Our results suggest that unlike most pore-forming proteins, Bax membrane permeabilization results from oligomerization of transmembrane monomers rather than concerted insertion of the pore domains of a preformed oligomer.
Figures



References
-
- Annis MG, Zamzami N, Zhu W, Penn LZ, Kroemer G, Leber B, Andrews DW (2001) Endoplasmic reticulum localized Bcl-2 prevents apoptosis when redistribution of cytochrome c is a late event. Oncogene 20: 1939–1952 - PubMed
-
- Belzacq AS, Vieira HL, Verrier F, Vandecasteele G, Cohen I, Prevost MC, Larquet E, Pariselli F, Petit PX, Kahn A, Rizzuto R, Brenner C, Kroemer G (2003) Bcl-2 and Bax modulate adenine nucleotide translocase activity. Cancer Res 63: 541–546 - PubMed
-
- Cartron PF, Priault M, Oliver L, Meflah K, Manon S, Vallette FM (2003) The N-terminal end of Bax contains a mitochondrial-targeting signal. J Biol Chem 278: 11633–11641 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

