EEDA: a protein associated with an early stage of stratified epithelial differentiation
- PMID: 15920738
- PMCID: PMC1523255
- DOI: 10.1002/jcp.20433
EEDA: a protein associated with an early stage of stratified epithelial differentiation
Abstract
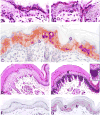
Using suppressive subtractive hybridization, we have identified a novel gene, which we named early epithelial differentiation associated (EEDA), which is uniquely associated with an early stage of stratified epithelial differentiation. In epidermis, esophageal epithelium, and tongue epithelium, EEDA mRNA, and antigen was abundant in suprabasal cells, but was barely detectable in more differentiated cells. Consistent with the limbal location of corneal epithelial stem cells, EEDA was expressed in basal corneal epithelial cells that are out of the stem cell compartment, as well as the suprabasal corneal epithelial cells. The strongest EEDA expression occurred in suprabasal precortical cells of mouse, bovine, and human anagen follicles. Developmental studies showed that the appearance of EEDA in embryonic mouse epidermis (E 15.5) coincided with morphological keratinization. Interestingly, EEDA expression is turned off when epithelia were perturbed by wounding and by cultivation under both low and high Ca2+ conditions. Our results indicate that EEDA is involved in the early stages of normal epithelial differentiation, and that EEDA is important for the "normal" differentiation pathway in a wide range of stratified epithelia.
Copyright 2005 Wiley-Liss, Inc.
Figures











Similar articles
-
Suprabasin, a novel epidermal differentiation marker and potential cornified envelope precursor.J Biol Chem. 2002 Nov 22;277(47):45195-202. doi: 10.1074/jbc.M205380200. Epub 2002 Sep 12. J Biol Chem. 2002. PMID: 12228223 Free PMC article.
-
CLED: a calcium-linked protein associated with early epithelial differentiation.Exp Cell Res. 2000 Aug 25;259(1):96-106. doi: 10.1006/excr.2000.4922. Exp Cell Res. 2000. PMID: 10942582
-
The bovine desmocollin family: a new gene and expression patterns reflecting epithelial cell proliferation and differentiation.J Cell Biol. 1994 Jul;126(2):507-18. doi: 10.1083/jcb.126.2.507. J Cell Biol. 1994. PMID: 8034749 Free PMC article.
-
Altered phenotype of cultured urothelial and other stratified epithelial cells: implications for wound healing.Am J Physiol Renal Physiol. 2006 Jul;291(1):F9-21. doi: 10.1152/ajprenal.00035.2006. Epub 2006 Apr 11. Am J Physiol Renal Physiol. 2006. PMID: 16609152 Review.
-
Epithelial stem cells: stepping out of their niche.Cell. 2004 Sep 3;118(5):530-2. doi: 10.1016/j.cell.2004.08.024. Cell. 2004. PMID: 15339656 Review.
Cited by
-
microRNA-103/107 Family Regulates Multiple Epithelial Stem Cell Characteristics.Stem Cells. 2015 May;33(5):1642-56. doi: 10.1002/stem.1962. Stem Cells. 2015. PMID: 25639731 Free PMC article.
-
Birt-Hogg-Dubé renal tumors are genetically distinct from other renal neoplasias and are associated with up-regulation of mitochondrial gene expression.BMC Med Genomics. 2010 Dec 16;3:59. doi: 10.1186/1755-8794-3-59. BMC Med Genomics. 2010. PMID: 21162720 Free PMC article.
-
Corneal epithelial biology: Lessons stemming from old to new.Exp Eye Res. 2020 Sep;198:108094. doi: 10.1016/j.exer.2020.108094. Epub 2020 Jul 19. Exp Eye Res. 2020. PMID: 32697979 Free PMC article. Review.
-
DAPL1 is a novel regulator of testosterone production in Leydig cells of mouse testis.Sci Rep. 2021 Sep 17;11(1):18532. doi: 10.1038/s41598-021-97961-6. Sci Rep. 2021. PMID: 34535743 Free PMC article.
-
Chronic Adrenergic Signaling Causes Abnormal RNA Expression of Proliferative Genes in Fetal Sheep Islets.Endocrinology. 2018 Oct 1;159(10):3565-3578. doi: 10.1210/en.2018-00540. Endocrinology. 2018. PMID: 30124804 Free PMC article.
References
-
- Asselineau D, Bernard BA, Bailly C, Darmon M, Prunieras M. Human epidermis reconstructed by culture: is it "normal"? J Invest Dermatol. 1986;86(2):181–186. - PubMed
-
- Cabodi S, Calautti E, Talora C, Kuroki T, Stein PL, Dotto GP. A PKC-eta/Fyn-dependent pathway leading to keratinocyte growth arrest and differentiation. Mol Cell. 2000;6(5):1121–1129. - PubMed
-
- Chaloin-Dufau C, Sun T-T, Dhouailly D. Appearance of the keratin pair K3/K12 during embryonic and adult corneal epithelial differentiation in the chick and in the rabbit. Cell Diff Dev. 1990;32:97–108. - PubMed
-
- Chen WY, Mui MM, Kao WW, Liu CY, Tseng SC. Conjunctival epithelial cells do not transdifferentiate in organotypic cultures: expression of K12 keratin is restricted to corneal epithelium. Current Eye Research. 1994;13(10):765–778. - PubMed
-
- Cotsarelis G, Cheng SZ, Dong G, Sun TT, Lavker RM. Existence of slow-cycling limbal epithelial basal cells that can be preferentially stimulated to proliferate: implications on epithelial stem cells. Cell. 1989;57(2):201–209. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

