Iron dysregulation combined with aging prevents sepsis-induced apoptosis
- PMID: 15921699
- PMCID: PMC1255961
- DOI: 10.1016/j.jss.2005.03.022
Iron dysregulation combined with aging prevents sepsis-induced apoptosis
Abstract
Background: Sepsis, iron loading, and aging cause independent increases in gut epithelial and splenic apoptosis. It is unknown how their combination will affect apoptosis and systemic cytokine levels.
Materials and methods: Hfe-/- mice (a murine homologue of hemochromatosis) abnormally accumulate iron in their tissues. Aged (24-26 months) or mature (16-18 months) Hfe-/- mice and wild type (WT) littermates were subjected to cecal ligation and puncture (CLP) or sham laparotomy. Intestine, spleen, and blood were harvested 24 h later and assessed for apoptosis and cytokine levels.
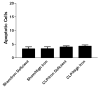
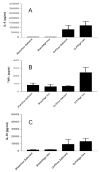
Results: Gut epithelial and splenic apoptosis were low in both aged septic and sham Hfe-/- mice, regardless of the amount of iron in their diet. Mature septic WT mice had increased apoptosis compared to age-matched sham WT mice. Mature septic Hfe-/- mice had similar levels of intestinal cell death to age-matched septic WT mice but higher levels of splenic apoptosis. Apoptosis was significantly lower in septic aged Hfe-/- mice than septic mature Hfe-/- animals. Interleukin-6 was elevated in septic aged Hfe-/- mice compared to sham mice.
Conclusions: Although sepsis, chronic iron dysregulation, and aging each increase gut and splenic apoptosis, their combination yields cell death levels similar to sham animals despite the fact that aged Hfe-/- mice are able to mount an inflammatory response following CLP and mature Hfe-/- mice have elevated sepsis-induced apoptosis. Combining sepsis with two risk factors that ordinarily increase cell death and increase mortality in CLP yields an apoptotic response that could not have been predicted based upon each element in isolation.
Figures





References
-
- Hotchkiss RS, Swanson PE, Cobb JP, Jacobson A, Buchman TG, Karl IE. Apoptosis in lymphoid and parenchymal cells during sepsis: findings in normal and T- and B-cell-deficient mice. Crit Care Med. 1997;25:1298–1307. - PubMed
-
- Hiramatsu M, Hotchkiss RS, Karl IE, Buchman TG. Cecal ligation and puncture (CLP) induces apoptosis in thymus, spleen, lung, and gut by an endotoxin and TNF-independent pathway. Shock. 1997;7:247–253. - PubMed
-
- Ayala A, Herdon CD, Lehman DL, Ayala CA, Chaudry IH. Differential induction of apoptosis in lymphoid tissues during sepsis: variation in onset, frequency, and the nature of the mediators. Blood. 1996;87:4261–4275. - PubMed
-
- Chung CS, Wang W, Chaudry IH, Ayala A. Increased apoptosis in lamina propria B cells during polymicrobial sepsis is FasL but not endotoxin mediated. Am J Physiol Gastrointest Liver Physiol. 2001;280:G812–G818. - PubMed
-
- Hotchkiss RS, Swanson PE, Freeman BD, Tinsley KW, Cobb JP, Matuschak GM, Buchman TG, Karl IE. Apoptotic cell death in patients with sepsis, shock, and multiple organ dysfunction. Crit Care Med. 1999;27:1230–1251. - PubMed
Publication types
MeSH terms
Grants and funding
- R01 GM044118/GM/NIGMS NIH HHS/United States
- K08 GM000709/GM/NIGMS NIH HHS/United States
- GM00709/GM/NIGMS NIH HHS/United States
- R01 GM066202/GM/NIGMS NIH HHS/United States
- GM48095/GM/NIGMS NIH HHS/United States
- GM08795/GM/NIGMS NIH HHS/United States
- R01 GM055194/GM/NIGMS NIH HHS/United States
- GM 44118/GM/NIGMS NIH HHS/United States
- P30 DK52574/DK/NIDDK NIH HHS/United States
- P30 DK052574/DK/NIDDK NIH HHS/United States
- R37 GM044118/GM/NIGMS NIH HHS/United States
- GM 66202/GM/NIGMS NIH HHS/United States
- GM 55194/GM/NIGMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

