Mammalian flavin-containing monooxygenases: structure/function, genetic polymorphisms and role in drug metabolism
- PMID: 15922018
- PMCID: PMC1828602
- DOI: 10.1016/j.pharmthera.2005.01.001
Mammalian flavin-containing monooxygenases: structure/function, genetic polymorphisms and role in drug metabolism
Abstract
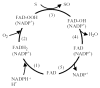
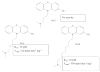
Flavin-containing monooxygenase (FMO) oxygenates drugs and xenobiotics containing a "soft-nucleophile", usually nitrogen or sulfur. FMO, like cytochrome P450 (CYP), is a monooxygenase, utilizing the reducing equivalents of NADPH to reduce 1 atom of molecular oxygen to water, while the other atom is used to oxidize the substrate. FMO and CYP also exhibit similar tissue and cellular location, molecular weight, substrate specificity, and exist as multiple enzymes under developmental control. The human FMO functional gene family is much smaller (5 families each with a single member) than CYP. FMO does not require a reductase to transfer electrons from NADPH and the catalytic cycle of the 2 monooxygenases is strikingly different. Another distinction is the lack of induction of FMOs by xenobiotics. In general, CYP is the major contributor to oxidative xenobiotic metabolism. However, FMO activity may be of significance in a number of cases and should not be overlooked. FMO and CYP have overlapping substrate specificities, but often yield distinct metabolites with potentially significant toxicological/pharmacological consequences. The physiological function(s) of FMO are poorly understood. Three of the 5 expressed human FMO genes, FMO1, FMO2 and FMO3, exhibit genetic polymorphisms. The most studied of these is FMO3 (adult human liver) in which mutant alleles contribute to the disease known as trimethylaminuria. The consequences of these FMO genetic polymorphisms in drug metabolism and human health are areas of research requiring further exploration.
Figures

References
-
- Akerman BR, Forrest S, Chow L, Youil R, Knight M, Treacy EP. Two novel mutations of the FMO3 gene in a proband with trimethylaminuria. Hum Mutat. 1999;13:376–379. - PubMed
-
- Akerman BR, Lemass H, Chow LM, Lambert DM, Greenberg C, Bibeau C, et al. Trimethylaminuria is caused by mutations of the FMO3 gene in a North American cohort. Mol Genet Metab. 1999;68:24–31. - PubMed
-
- Atta-Asafo-Adjei E, Lawton MP, Philpot RM. Cloning, sequencing, distribution, and expression in Escherichia coli of flavin-containing monooxygenase 1C1. Evidence for a third gene subfamiliy in rabbits. J Biol Chem. 1993;268:9681–9689. - PubMed
-
- Attar M, Dong D, Ling KH, Tang-Liu DD. Cytochrome P450 2C8 and flavin-containing monooxygenases are involved in the metabolism of tazarotenic acid in humans. Drug Metab Dispos. 2003;31:476–481. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

