Ras diffusion is sensitive to plasma membrane viscosity
- PMID: 15923235
- PMCID: PMC1366624
- DOI: 10.1529/biophysj.104.055640
Ras diffusion is sensitive to plasma membrane viscosity
Abstract
The cell surface contains a variety of barriers and obstacles that slow the lateral diffusion of glycosylphosphatidylinositol (GPI)-anchored and transmembrane proteins below the theoretical limit imposed by membrane viscosity. How the diffusion of proteins residing exclusively on the inner leaflet of the plasma membrane is regulated has been largely unexplored. We show here that the diffusion of the small GTPase Ras is sensitive to the viscosity of the plasma membrane. Using confocal fluorescence recovery after photobleaching, we examined the diffusion of green fluorescent protein (GFP)-tagged HRas, NRas, and KRas in COS-7 cells loaded with or depleted of cholesterol, a well-known modulator of membrane bilayer viscosity. In cells loaded with excess cholesterol, the diffusional mobilities of GFP-HRas, GFP-NRas, and GFP-KRas were significantly reduced, paralleling the behavior of the viscosity-sensitive lipid probes DiIC(16) and DiIC(18). However, the effects of cholesterol depletion on protein and lipid diffusion in cell membranes were highly dependent on the depletion method used. Cholesterol depletion with methyl-beta-cyclodextrin slowed Ras diffusion by a viscosity-independent mechanism, whereas overnight cholesterol depletion slightly increased both protein and lipid diffusion. The ability of Ras to sense membrane viscosity may represent a general feature of proteins residing on the cytoplasmic face of the plasma membrane.
Figures
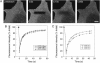


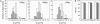
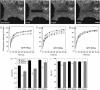


References
-
- Jacobson, K., A. Ishihara, and R. Inman. 1987. Lateral diffusion of proteins in membranes. Annu. Rev. Physiol. 49:163–175. - PubMed
-
- Edidin, M. 1996. Getting there is only half the fun. Curr. Top. Membr. 43:1–13.
-
- Edidin, M. 2001. Shrinking patches and slippery rafts: scales of domains in the plasma membrane. Trends Cell Biol. 11:492–496. - PubMed
-
- Kusumi, A., and Y. Sako. 1996. Cell surface organization by the membrane skeleton. Curr. Opin. Cell Biol. 8:566–574. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

