Molecularly engineered PEG hydrogels: a novel model system for proteolytically mediated cell migration
- PMID: 15923238
- PMCID: PMC1366622
- DOI: 10.1529/biophysj.104.050682
Molecularly engineered PEG hydrogels: a novel model system for proteolytically mediated cell migration
Abstract
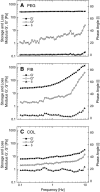
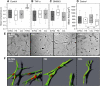
Model systems mimicking the extracellular matrix (ECM) have greatly helped in quantifying cell migration in three dimensions and elucidated the molecular determinants of cellular motility in morphogenesis, regeneration, and disease progression. Here we tested the suitability of proteolytically degradable synthetic poly(ethylene glycol) (PEG)-based hydrogels as an ECM model system for cell migration research and compared this designer matrix with the two well-established ECM mimetics fibrin and collagen. Three-dimensional migration of dermal fibroblasts was quantified by time-lapse microscopy and automated single-cell tracking. A broadband matrix metalloproteinase (MMP) inhibitor and tumor necrosis factor-alpha, a potent MMP-inducer in fibroblasts, were used to alter MMP regulation. We demonstrate a high sensitivity of migration in synthetic networks to both MMP modulators: inhibition led to an almost complete suppression of migration in PEG hydrogels, whereas MMP upregulation increased the fraction of migrating cells significantly. Conversely, migration in collagen and fibrin proved to be less sensitive to the above MMP modulators, as their fibrillar architecture allowed for MMP-independent migration through preexisting pores. The possibility of molecularly recapitulating key functions of the natural extracellular microenvironment and the improved protease sensitivity makes PEG hydrogels an interesting model system that allows correlation between protease activity and cell migration.
Figures





References
-
- Ridley, A. J., M. A. Schwartz, K. Burridge, R. A. Firtel, M. H. Ginsberg, G. Borisy, J. T. Parsons, and A. R. Horwitz. 2003. Cell migration: integrating signals from front to back. Science. 302:1704–1709. - PubMed
-
- Lauffenburger, D. A., and A. F. Horwitz. 1996. Cell migration: a physically integrated molecular process. Cell. 84:359–369. - PubMed
-
- Friedl, P., and K. Wolf. 2003. Tumour-cell invasion and migration: diversity and escape mechanisms. Nat. Rev. Cancer. 3:362–374. - PubMed
-
- Wolf, K., R. Muller, S. Borgmann, E. B. Brocker, and P. Friedl. 2003. Amoeboid shape change and contact guidance: T-lymphocyte crawling through fibrillar collagen is independent of matrix remodeling by MMPs and other proteases. Blood. 102:3262–3269. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

