Heptameric (L12)6/L10 rather than canonical pentameric complexes are found by tandem MS of intact ribosomes from thermophilic bacteria
- PMID: 15923259
- PMCID: PMC1149426
- DOI: 10.1073/pnas.0502193102
Heptameric (L12)6/L10 rather than canonical pentameric complexes are found by tandem MS of intact ribosomes from thermophilic bacteria
Abstract
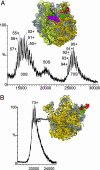
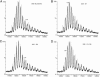
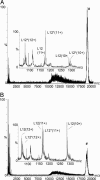
Ribosomes are universal translators of the genetic code into protein and represent macromolecular structures that are asymmetric, often heterogeneous, and contain dynamic regions. These properties pose considerable challenges for modern-day structural biology. Despite these obstacles, high-resolution x-ray structures of the 30S and 50S subunits have revealed the RNA architecture and its interactions with proteins for ribosomes from Thermus thermophilus, Deinococcus radiodurans, and Haloarcula marismortui. Some regions, however, remain inaccessible to these high-resolution approaches because of their high conformational dynamics and potential heterogeneity, specifically the so-called L7/L12 stalk complex. This region plays a vital role in protein synthesis by interacting with GTPase factors in translation. Here, we apply tandem MS, an approach widely applied to peptide sequencing for proteomic applications but not previously applied to MDa complexes. Isolation and activation of ions assigned to intact 30S and 50S subunits releases proteins S6 and L12, respectively. Importantly, this process reveals, exclusively while attached to ribosomes, a phosphorylation of L12, the protein located in multiple copies at the tip of the stalk complex. Moreover, through tandem MS we discovered a stoichiometry for the stalk protuberance on Thermus thermophilus and other thermophiles and contrast this assembly with the analogous one on ribosomes from mesophiles. Together with evidence for a potential interaction with the degradosome, these results show that important findings on ribosome structure, interactions, and modifications can be discovered by tandem MS, even on well studied ribosomes from Thermus thermophilus.
Figures

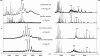
References
-
- Wimberly, B. T., Brodersen, D. E., Clemons, W. M., Jr., Morgan-Warren, R. J., Carter, A. P., Vonrhein, C., Hartsch, T. & Ramakrishnan, V. (2000) Nature 407, 327-339. - PubMed
-
- Schluenzen, F., Tocilj, A., Zarivach, R., Harms, J., Gluehmann, M., Janell, D., Bashan, A., Bartels, H., Agmon, I., Franceschi, F. & Yonath, A. (2000) Cell 102, 615-623. - PubMed
-
- Harms, J., Schluenzen, F., Zarivach, R., Bashan, A., Gat, S., Agmon, I., Bartels, H., Franceschi, F. & Yonath, A. (2001) Cell 107, 679-688. - PubMed
-
- Ban, N., Nissen, P., Hansen, J., Moore, P. B. & Steitz, T. A. (2000) Science 289, 905-920. - PubMed
-
- Gudkov, A. T., Tumanova, L. G., Venyaminov, S. Y. & Khechinashvilli, N. N. (1978) FEBS Lett. 93, 215-218. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

