Heat stress phenotypes of Arabidopsis mutants implicate multiple signaling pathways in the acquisition of thermotolerance
- PMID: 15923322
- PMCID: PMC1150405
- DOI: 10.1104/pp.105.062257
Heat stress phenotypes of Arabidopsis mutants implicate multiple signaling pathways in the acquisition of thermotolerance
Abstract
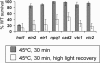
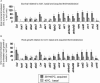
To investigate the importance of different processes to heat stress tolerance, 45 Arabidopsis (Arabidopsis thaliana) mutants and one transgenic line were tested for basal and acquired thermotolerance at different stages of growth. Plants tested were defective in signaling pathways (abscisic acid, salicylic acid, ethylene, and oxidative burst signaling) and in reactive oxygen metabolism (ascorbic acid or glutathione production, catalase) or had previously been found to have temperature-related phenotypes (e.g. fatty acid desaturase mutants, uvh6). Mutants were assessed for thermotolerance defects in seed germination, hypocotyl elongation, root growth, and seedling survival. To assess oxidative damage and alterations in the heat shock response, thiobarbituric acid reactive substances, heat shock protein 101, and small heat shock protein levels were determined. Fifteen mutants showed significant phenotypes. Abscisic acid (ABA) signaling mutants (abi1 and abi2) and the UV-sensitive mutant, uvh6, showed the strongest defects in acquired thermotolerance of root growth and seedling survival. Mutations in nicotinamide adenine dinucleotide phosphate oxidase homolog genes (atrbohB and D), ABA biosynthesis mutants (aba1, aba2, and aba3), and NahG transgenic lines (salicylic acid deficient) showed weaker defects. Ethylene signaling mutants (ein2 and etr1) and reactive oxygen metabolism mutants (vtc1, vtc2, npq1, and cad2) were more defective in basal than acquired thermotolerance, especially under high light. All mutants accumulated wild-type levels of heat shock protein 101 and small heat shock proteins. These data indicate that, separate from heat shock protein induction, ABA, active oxygen species, and salicylic acid pathways are involved in acquired thermotolerance and that UVH6 plays a significant role in temperature responses in addition to its role in UV stress.
Figures





References
-
- Alfonso M, Yruela I, Almarcegui S, Torrado E, Perez MA, Picorel R (2001) Unusual tolerance to high temperatures in a new herbicide-resistant D1 mutant from Glycine max (L.) Merr. cell cultures deficient in fatty acid desaturation. Planta 212: 573–582 - PubMed
-
- Argandona VH, Chaman M, Cardemil L, Munoz O, Zuniga GE, Corcuera LJ (2001) Ethylene production and peroxidase activity in aphid-infested barley. J Chem Ecol 27: 53–68 - PubMed
-
- Berardini TZ, Bollman K, Sun H, Poethig RS (2001) Regulation of vegetative phase change in Arabidopsis thaliana by cyclophilin 40. Science 291: 2405–2407 - PubMed
-
- Bortier K, Dekelver G, De Temmerman L, Ceulemans R (2001) Stem injection of Populus nigra with EDU to study ozone effects under field conditions. Environ Pollut 111: 199–208 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

