Progestins induce transcriptional activation of signal transducer and activator of transcription 3 (Stat3) via a Jak- and Src-dependent mechanism in breast cancer cells
- PMID: 15923602
- PMCID: PMC1140598
- DOI: 10.1128/MCB.25.12.4826-4840.2005
Progestins induce transcriptional activation of signal transducer and activator of transcription 3 (Stat3) via a Jak- and Src-dependent mechanism in breast cancer cells
Abstract
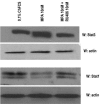
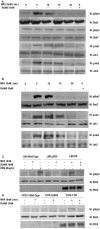
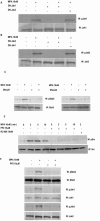
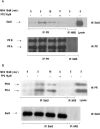
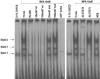
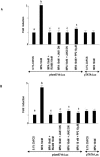
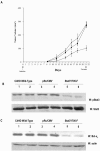
Interactions between steroid hormone receptors and signal transducer and activator of transcription (Stat)-mediated signaling pathways have already been described. In the present study, we explored the capacity of progestins to modulate Stat3 transcriptional activation in an experimental model of hormonal carcinogenesis in which the synthetic progestin medroxyprogesterone acetate (MPA) induced mammary adenocarcinomas in BALB/c mice and in the human breast cancer cell line T47D. We found that C4HD epithelial cells, from the MPA-induced mammary tumor model, expressed Stat3 and that MPA treatment of C4HD cells up-regulated Stat3 protein expression. In addition, MPA induced rapid, nongenomic Stat3, Jak1, and Jak2 tyrosine phosphorylation in C4HD and T47D cells. MPA treatment of C4HD cells also resulted in rapid c-Src tyrosine phosphorylation. These effects were completely abolished by the progestin antagonist RU486. Abrogation of Jak1 and Jak2 activity by transient transfection of C4HD cells with dominant negative (DN) Jak1 or DN Jak2 vectors, or inhibition of Src activity by preincubation of cells with the Src family kinase inhibitor PP2, blocked the capacity of MPA to induce Stat3 phosphorylation. Treatment of C4HD cells with MPA induced Stat3 binding to DNA. In addition, MPA promoted strong Stat3 transcriptional activation in C4HD and T47D cells that was inhibited by RU486 and by blockage of Jak1, Jak2, and Src activities. To investigate the correlation between MPA-induced Stat3 activation and cell growth, C4HD cells were transiently transfected with a DN Stat3 expression vector, Stat3Y705-F, or with a constitutively activated Stat3 mutant, Stat3-C. While expression of Stat3Y705-F mutant had an inhibitory effect on MPA-induced growth of C4HD cells, transfection with the constitutively activated Stat3-C vector resulted in MPA-independent proliferation. Finally, we addressed the effect of targeting Stat3 in in vivo growth of C4HD breast tumors. Blockage of Stat3 activation by transfection of C4HD cells with the DN Stat3Y705-F expression vector significantly inhibited these cells' ability to form tumors in syngeneic mice. Our results have for the first time demonstrated that progestins are able to induce Stat3 transcriptional activation, which is in turn an obligatory requirement for progestin stimulation of both in vitro and in vivo breast cancer growth.
Figures


References
-
- Aaronson, D. S., and C. M. Horvath. 2002. A road map for those who don't know JAK-STAT. Science 296:1653-1655. - PubMed
-
- Amin, H. M., T. J. McDonnell, Y. Ma, Q. Lin, Y. Fujio, K. Kunisada, V. Leventaki, P. Das, G. Z. Rassidakis, C. Cutler, L. J. Medeiros, and R. Lai. 2004. Selective inhibition of STAT3 induces apoptosis and G1 cell cycle arrest in ALK-positive anaplastic large cell lymphoma. Oncogene 23:5426-5434. - PubMed
-
- Balana, M. E., L. Labriola, M. Salatino, F. Movsichoff, G. Peters, E. H. Charreau, and P. V. Elizalde. 2001. Activation of ErbB-2 via a hierarchical interaction between ErbB-2 and type I insulin-like growth factor receptor in mammary tumor cells. Oncogene 20:34-47. - PubMed
-
- Balana, M. E., R. Lupu, L. Labriola, E. H. Charreau, and P. V. Elizalde. 1999. Interactions between progestins and heregulin (HRG) signaling pathways: HRG acts as mediator of progestins proliferative effects in mouse mammary adenocarcinomas. Oncogene 18:6370-6379. - PubMed
-
- Ballare, C., M. Uhrig, T. Bechtold, E. Sancho, M. Di Domenico, A. Migliaccio, F. Auricchio, and M. Beato. 2003. Two domains of the progesterone receptor interact with the estrogen receptor and are required for progesterone activation of the c-Src/Erk pathway in mammalian cells. Mol. Cell. Biol. 23:1994-2008. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous
