Mitochondrial reactive oxygen species activation of p38 mitogen-activated protein kinase is required for hypoxia signaling
- PMID: 15923604
- PMCID: PMC1140591
- DOI: 10.1128/MCB.25.12.4853-4862.2005
Mitochondrial reactive oxygen species activation of p38 mitogen-activated protein kinase is required for hypoxia signaling
Abstract
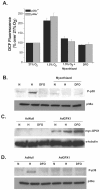
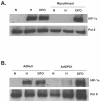
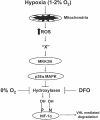
Mammalian cells have the ability to sense low oxygen levels (hypoxia). An adaptive response to hypoxia involves the induction of the transcription factor hypoxia-inducible factor 1 (HIF-1). The intracellular signaling pathways that regulate HIF-1 activation during hypoxia remain unknown. Here, we demonstrate that p38alpha-/- cells fail to activate HIF-1 under hypoxic conditions. Cells deficient in Mkk3 and Mkk6, the upstream regulators of p38alpha, also fail to activate HIF-1 under hypoxic conditions. The p38alpha-/- cells are able to activate HIF-1 in response to anoxia or iron chelators during normoxia. Furthermore, the hypoxic activation of p38alpha and HIF-1 was abolished by myxothiazol, a mitochondrial complex III inhibitor, and glutathione peroxidase 1 (GPX1), a scavenger of hydrogen peroxide. Thus, the activation of p38alpha and HIF-1 is dependent on the generation of mitochondrial reactive oxygen species. These results provide genetic evidence that p38 mitogen-activated protein kinase signaling is essential for HIF-1 activation.
Figures
Similar articles
-
Multiple activation mechanisms of p38alpha mitogen-activated protein kinase.J Biol Chem. 2006 Sep 8;281(36):26225-34. doi: 10.1074/jbc.M606800200. Epub 2006 Jul 18. J Biol Chem. 2006. PMID: 16849316
-
Activation of mitogen-activated protein kinase kinase (MKK) 3 and MKK6 by type I interferons.J Biol Chem. 2005 Mar 18;280(11):10001-10. doi: 10.1074/jbc.M410972200. Epub 2005 Jan 11. J Biol Chem. 2005. PMID: 15644321
-
The Kaposi's sarcoma-associated herpes virus G protein-coupled receptor up-regulates vascular endothelial growth factor expression and secretion through mitogen-activated protein kinase and p38 pathways acting on hypoxia-inducible factor 1alpha.Cancer Res. 2000 Sep 1;60(17):4873-80. Cancer Res. 2000. PMID: 10987301
-
Transduction pathways involved in Hypoxia-Inducible Factor-1 phosphorylation and activation.Free Radic Biol Med. 2001 Oct 1;31(7):847-55. doi: 10.1016/s0891-5849(01)00657-8. Free Radic Biol Med. 2001. PMID: 11585703 Review.
-
Regulation of the hypoxia-inducible transcription factor HIF-1 by reactive oxygen species in smooth muscle cells.Adv Exp Med Biol. 2003;536:171-8. doi: 10.1007/978-1-4419-9280-2_22. Adv Exp Med Biol. 2003. PMID: 14635664 Review. No abstract available.
Cited by
-
Vav1 is Essential for HIF-1α Activation via a Lysosomal VEGFR1-Mediated Degradation Mechanism in Endothelial Cells.Cancers (Basel). 2020 May 27;12(6):1374. doi: 10.3390/cancers12061374. Cancers (Basel). 2020. PMID: 32471123 Free PMC article.
-
Training in the fasted state facilitates re-activation of eEF2 activity during recovery from endurance exercise.Eur J Appl Physiol. 2011 Jul;111(7):1297-305. doi: 10.1007/s00421-010-1753-7. Epub 2010 Dec 4. Eur J Appl Physiol. 2011. PMID: 21132439 Clinical Trial.
-
D-Glucosamine supplementation extends life span of nematodes and of ageing mice.Nat Commun. 2014 Apr 8;5:3563. doi: 10.1038/ncomms4563. Nat Commun. 2014. PMID: 24714520 Free PMC article.
-
Signal integration by JNK and p38 MAPK pathways in cancer development.Nat Rev Cancer. 2009 Aug;9(8):537-49. doi: 10.1038/nrc2694. Nat Rev Cancer. 2009. PMID: 19629069 Review.
-
Macrophage Motility in Wound Healing Is Regulated by HIF-1α via S1P Signaling.Int J Mol Sci. 2021 Aug 20;22(16):8992. doi: 10.3390/ijms22168992. Int J Mol Sci. 2021. PMID: 34445695 Free PMC article. Review.
References
-
- Adams, R. H., A. Porras, G. Alonso, M. Jones, K. Vintersten, S. Panelli, A. Valladares, L. Perez, R. Klein, and A. R. Nebreda. 2000. Essential role of p38alpha MAP kinase in placental but not embryonic cardiovascular development. Mol. Cell 6:109-116. - PubMed
-
- Aragones, J., D. R. Jones, S. Martin, M. A. San Juan, A. Alfranca, F. Vidal, A. Vara, I. Merida, and M. O. Landazuri. 2001. Evidence for the involvement of diacylglycerol kinase in the activation of hypoxia-inducible transcription factor 1 by low oxygen tension. J. Biol. Chem. 276:10548-10555. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous
