Reduced affinity to and inhibition by DKK1 form a common mechanism by which high bone mass-associated missense mutations in LRP5 affect canonical Wnt signaling
- PMID: 15923613
- PMCID: PMC1140571
- DOI: 10.1128/MCB.25.12.4946-4955.2005
Reduced affinity to and inhibition by DKK1 form a common mechanism by which high bone mass-associated missense mutations in LRP5 affect canonical Wnt signaling
Abstract
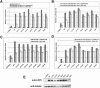
The low-density-lipoprotein receptor-related protein 5 (LRP5), a coreceptor in the canonical Wnt signaling pathway, has been implicated in human disorders of low and high bone mass. Loss-of-function mutations cause the autosomal recessive osteoporosis-pseudoglioma syndrome, and heterozygous missense mutations in families segregating autosomal dominant high bone mass (HBM) phenotypes have been identified. We expressed seven different HBM-LRP5 missense mutations to delineate the mechanism by which they alter Wnt signaling. None of the mutations caused activation of the receptor in the absence of ligand. Each mutant receptor was able to reach the cell surface, albeit at differing amounts, and transduce exogenously supplied Wnt1 and Wnt3a signal. All HBM mutant proteins had reduced physical interaction with and reduced inhibition by DKK1. These data suggest that HBM mutant proteins can transit to the cell surface in sufficient quantity to transduce Wnt signal and that the likely mechanism for the HBM mutations' physiologic effects is via reduced affinity to and inhibition by DKK1.
Figures



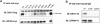
References
-
- Bafico, A., G. Liu, A. Yaniv, A. Gazit, and S. A. Aaronson. 2001. Novel mechanism of Wnt signalling inhibition mediated by Dickkopf-1 interaction with LRP6/Arrow. Nat. Cell Biol. 3:683-686. - PubMed
-
- Bodine, P. V., W. Zhao, Y. P. Kharode, F. J. Bex, A. J. Lambert, M. B. Goad, T. Gaur, G. S. Stein, J. B. Lian, and B. S. Komm. 2004. The Wnt antagonist secreted frizzled-related protein-1 is a negative regulator of trabecular bone formation in adult mice. Mol. Endocrinol. 18:1222-1237. - PubMed
-
- Boyden, L. M., J. Mao, J. Belsky, L. Mitzner, A. Farhi, M. A. Mitnick, D. Wu, K. Insogna, and R. P. Lifton. 2002. High bone density due to a mutation in LDL-receptor-related protein 5. N. Engl. J. Med. 346:1513-1521. - PubMed
-
- Fujino, T., H. Asaba, M. J. Kang, Y. Ikeda, H. Sone, S. Takada, D. H. Kim, R. X. Ioka, M. Ono, H. Tomoyori, M. Okubo, T. Murase, A. Kamataki, J. Yamamoto, K. Magoori, S. Takahashi, Y. Miyamoto, H. Oishi, M. Nose, M. Okazaki, S. Usui, K. Imaizumi, M. Yanagisawa, J. Sakai, and T. T. Yamamoto. 2003. Low-density lipoprotein receptor-related protein 5 (LRP5) is essential for normal cholesterol metabolism and glucose-induced insulin secretion. Proc. Natl. Acad. Sci. USA 100:229-234. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
