DNA topoisomerase I is a cofactor for c-Jun in the regulation of epidermal growth factor receptor expression and cancer cell proliferation
- PMID: 15923621
- PMCID: PMC1140586
- DOI: 10.1128/MCB.25.12.5040-5051.2005
DNA topoisomerase I is a cofactor for c-Jun in the regulation of epidermal growth factor receptor expression and cancer cell proliferation
Abstract
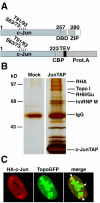
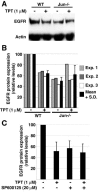
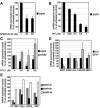
DNA topoisomerase I (Topo I) is a molecular target for the anticancer agent topotecan in the treatment of small cell lung cancer and ovarian carcinomas. However, the molecular mechanisms by which topotecan treatment inhibits cancer cell proliferation are unclear. We describe here the identification of Topo I as a novel endogenous interaction partner for transcription factor c-Jun. Reciprocal coimmunoprecipitation analysis showed that Topo I and c-Jun interact in transformed human cells in a manner that is dependent on JNK activity. c-Jun target gene epidermal growth factor receptor (EGFR) was identified as a novel gene whose expression was specifically inhibited by topotecan. Moreover, Topo I overexpression supported c-Jun-mediated reporter gene activation and both genetic and chemical inhibition of c-Jun converted cells resistant to topotecan-elicited EGFR downregulation. Topotecan-elicited suppression of proliferation was rescued by exogenously expressed EGFR. Furthermore, we demonstrate the cooperation of the JNK-c-Jun pathway, Topo I, and EGFR in the positive regulation of HT-1080 cell proliferation. Together, these results have identified transcriptional coactivator Topo I as a first endogenous cofactor for c-Jun in the regulation of cell proliferation. In addition, the results of the present study strongly suggest that inhibition of EGFR expression is a novel mechanism by which topotecan inhibits cell proliferation in cancer therapy.
Figures

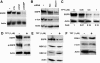


Similar articles
-
Pharmacodynamics of topoisomerase I inhibition: Western blot determination of topoisomerase I and cleavable complex in patients with upper gastrointestinal malignancies treated with topotecan.Clin Cancer Res. 1998 Mar;4(3):545-57. Clin Cancer Res. 1998. PMID: 9533521 Clinical Trial.
-
Retinoic acid inhibits induction of c-Jun protein by ultraviolet radiation that occurs subsequent to activation of mitogen-activated protein kinase pathways in human skin in vivo.J Clin Invest. 1998 Mar 15;101(6):1432-40. doi: 10.1172/JCI2153. J Clin Invest. 1998. PMID: 9502786 Free PMC article.
-
Antitumor activity of sequential treatment with topotecan and anti-epidermal growth factor receptor monoclonal antibody C225.Clin Cancer Res. 1999 Apr;5(4):909-16. Clin Cancer Res. 1999. PMID: 10213228
-
Epidermal Growth Factor Receptor (EGFR) and its Cross-Talks with Topoisomerases: Challenges and Opportunities for Multi-Target Anticancer Drugs.Curr Pharm Des. 2016;22(21):3226-36. doi: 10.2174/1381612822666160224142200. Curr Pharm Des. 2016. PMID: 26907945 Review.
-
A pharmacokinetic evaluation of topotecan as a cervical cancer therapy.Expert Opin Drug Metab Toxicol. 2013 Feb;9(2):215-24. doi: 10.1517/17425255.2013.758249. Expert Opin Drug Metab Toxicol. 2013. PMID: 23320990 Review.
Cited by
-
Human cancer cell line microRNAs associated with in vitro sensitivity to paclitaxel.Oncol Rep. 2014 Jan;31(1):376-83. doi: 10.3892/or.2013.2847. Epub 2013 Nov 13. Oncol Rep. 2014. PMID: 24220856 Free PMC article.
-
Inhibition of prostate cancer cell line (PC-3) by anhydrodihydroartemisinin (ADHA) through caspase-dependent pathway.EXCLI J. 2020 May 11;19:613-619. doi: 10.17179/excli2020-1331. eCollection 2020. EXCLI J. 2020. PMID: 32483407 Free PMC article.
-
MAP3K1 and MAP2K4 mutations are associated with sensitivity to MEK inhibitors in multiple cancer models.Cell Res. 2018 Jul;28(7):719-729. doi: 10.1038/s41422-018-0044-4. Epub 2018 May 24. Cell Res. 2018. PMID: 29795445 Free PMC article.
-
Natural Compounds as Anticancer Agents Targeting DNA Topoisomerases.Curr Genomics. 2017 Feb;18(1):75-92. doi: 10.2174/1389202917666160808125213. Curr Genomics. 2017. PMID: 28503091 Free PMC article. Review.
-
Epidermal Growth Factor Receptor Cell Proliferation Signaling Pathways.Cancers (Basel). 2017 May 17;9(5):52. doi: 10.3390/cancers9050052. Cancers (Basel). 2017. PMID: 28513565 Free PMC article. Review.
References
-
- Basehoar, A. D., S. J. Zanton, and F. B. Pugh. 2004. Identification and distinct regulation of yeast TATA box-containing genes. Cell 116:699-709. - PubMed
-
- Blaney, S. M., D. E. Cole, F. M. Balis, K. Godwin, and D. G. Poplack. 1993. Plasma and cerebrospinal fluid pharmacokinetic study of topotecan in nonhuman primates. Cancer Res. 53:725-727. - PubMed
-
- Brummelkamp, T. R., R. Bernards, and R. Agami. 2002. A system for stable expression of short interfering RNAs in mammalian cells. Science 296:550-553. - PubMed
-
- Daoud, S. S., P. J. Munson, W. Reinhold, L. Young, V. V. Prabhu, Q. Yu, J. LaRose, K. W. Kohn, J. N. Weinstein, and Y. Pommier. 2003. Impact of p53 knockout and topotecan treatment on gene expression profiles in human colon carcinoma cells: a pharmacogenomic study. Cancer Res. 63:2782-2793. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
