Connexin 40, a target of transcription factor Tbx5, patterns wrist, digits, and sternum
- PMID: 15923624
- PMCID: PMC1140596
- DOI: 10.1128/MCB.25.12.5073-5083.2005
Connexin 40, a target of transcription factor Tbx5, patterns wrist, digits, and sternum
Abstract
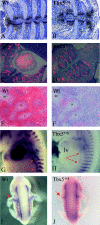
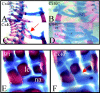
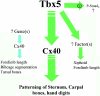
Haploinsufficiency of T-box transcription factor 5 (TBX5) causes human Holt-Oram syndrome (HOS), a developmental disorder characterized by skeletal and heart malformations. Mice carrying a Tbx5 null allele (Tbx5(+/Delta)) have malformations in digits, wrists, and sternum joints, regions where Tbx5 is expressed. We demonstrate that mice deficient in connexin 40 (Cx40), a Tbx5-regulated gap junction component, shared axial and appendicular skeletal malformations with Tbx5(+/Delta) mice. Although no role in skeleton patterning has been described for gap junctions, we demonstrate here that Cx40 is involved in formation of specific joints, as well as bone shape. Even a 50% reduction in either Tbx5 or Cx40 produces bone abnormalities, demonstrating their crucial control over skeletal development. Further, we demonstrate that Tbx5 exerts in part its key regulatory role in bone growth and maturation by controlling via Cx40 the expression of Sox9 (a transcription factor essential for chondrogenesis and skeleton growth). Our study strongly suggests that Cx40 deficiency accounts for many skeletal malformations in HOS and that Tbx5 regulation of Cx40 plays a critical role in the exquisite developmental patterning of the forelimbs and sternum.
Figures



References
-
- Agarwal, P., J. N. Wylie, J. Galceran, O. Arkhitko, C. Li, C. Deng, R. Grosschedl, and B. G. Bruneau. 2003. Tbx5 is essential for forelimb bud initiation following patterning of the limb field in the mouse embryo. Development 130:623-633. - PubMed
-
- Ausubel, F., R. Brent, R. E. Kingston, D. D. Moore, J. G. Seidman, J. A. Smith, and K. Struhl. (ed.). 2004. Current protocols in molecular biology. John Wiley & Sons, Inc., New York, N.Y.
-
- Baitner, A. C., S. G. Maurer, M. B. Gruen, and P. E. Di Cesare. 2000. The genetic basis of the osteochondrodysplasias. J. Pediatr. Orthop. 20:594-605. - PubMed
-
- Bamshad, M., R. C. Lin, D. J. Law, W. C. Watkins, P. A. Krakowiak, M. E. Moore, P. Franceschini, R. Lala, L. B. Holmes, T. C. Gebuhr, B. G. Bruneau, A. Schinzel, J. G. Seidman, C. E. Seidman, and L. B. Jorde. 1997. Mutations in human TBX3 alter limb, apocrine and genital development in ulnar-mammary syndrome. Nat. Genet. 16:311-315. [Erratum, 19(1): 102, 1998.] - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous
