The MEK1 scaffolding protein MP1 regulates cell spreading by integrating PAK1 and Rho signals
- PMID: 15923628
- PMCID: PMC1140582
- DOI: 10.1128/MCB.25.12.5119-5133.2005
The MEK1 scaffolding protein MP1 regulates cell spreading by integrating PAK1 and Rho signals
Abstract
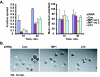
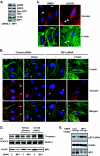
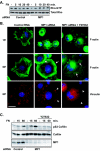
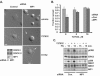
How the extracellular signal-regulated kinase (ERK) cascade regulates diverse cellular functions, including cell proliferation, survival, and motility, in a context-dependent manner remains poorly understood. Compelling evidence indicates that scaffolding molecules function in yeast to channel specific signals through common components to appropriate targets. Although a number of putative ERK scaffolding proteins have been identified in mammalian systems, none has been linked to a specific biological response. Here we show that the putative scaffold protein MEK partner 1 (MP1) and its partner p14 regulate PAK1-dependent ERK activation during adhesion and cell spreading but are not required for ERK activation by platelet-derived growth factor. MP1 associates with active but not inactive PAK1 and controls PAK1 phosphorylation of MEK1. Our data further show that MP1, p14, and MEK1 serve to inhibit Rho/Rho kinase functions necessary for the turnover of adhesion structures and cell spreading and reveal a signal-channeling function for a MEK1/ERK scaffold in orchestrating cytoskeletal rearrangements important for cell motility.
Figures



Similar articles
-
Extracellular signal-regulated kinase promotes Rho-dependent focal adhesion formation by suppressing p190A RhoGAP.Mol Cell Biol. 2010 Jul;30(13):3233-48. doi: 10.1128/MCB.01178-09. Epub 2010 May 3. Mol Cell Biol. 2010. PMID: 20439493 Free PMC article.
-
p14-MP1-MEK1 signaling regulates endosomal traffic and cellular proliferation during tissue homeostasis.J Cell Biol. 2006 Dec 18;175(6):861-8. doi: 10.1083/jcb.200607025. J Cell Biol. 2006. PMID: 17178906 Free PMC article.
-
PAK1 phosphorylation of MEK1 regulates fibronectin-stimulated MAPK activation.J Cell Biol. 2003 Jul 21;162(2):281-91. doi: 10.1083/jcb.200212141. J Cell Biol. 2003. PMID: 12876277 Free PMC article.
-
Regulation of phosphorylation pathways by p21 GTPases. The p21 Ras-related Rho subfamily and its role in phosphorylation signalling pathways.Eur J Biochem. 1996 Dec 1;242(2):171-85. doi: 10.1111/j.1432-1033.1996.0171r.x. Eur J Biochem. 1996. PMID: 8973630 Review.
-
The regulation of extracellular signal-regulated kinase (ERK) in mammalian cells.Int J Biochem Cell Biol. 2008;40(12):2707-19. doi: 10.1016/j.biocel.2008.04.009. Epub 2008 May 15. Int J Biochem Cell Biol. 2008. PMID: 18562239 Review.
Cited by
-
RACK1 targets the extracellular signal-regulated kinase/mitogen-activated protein kinase pathway to link integrin engagement with focal adhesion disassembly and cell motility.Mol Cell Biol. 2007 Dec;27(23):8296-305. doi: 10.1128/MCB.00598-07. Epub 2007 Oct 1. Mol Cell Biol. 2007. PMID: 17908799 Free PMC article.
-
Signaling dynamics of the KSR1 scaffold complex.Proc Natl Acad Sci U S A. 2009 Jul 7;106(27):11022-7. doi: 10.1073/pnas.0901590106. Epub 2009 Jun 18. Proc Natl Acad Sci U S A. 2009. PMID: 19541618 Free PMC article.
-
LAMTOR2-mediated modulation of NGF/MAPK activation kinetics during differentiation of PC12 cells.PLoS One. 2014 Apr 21;9(4):e95863. doi: 10.1371/journal.pone.0095863. eCollection 2014. PLoS One. 2014. PMID: 24752675 Free PMC article.
-
Extracellular signal-regulated kinase promotes Rho-dependent focal adhesion formation by suppressing p190A RhoGAP.Mol Cell Biol. 2010 Jul;30(13):3233-48. doi: 10.1128/MCB.01178-09. Epub 2010 May 3. Mol Cell Biol. 2010. PMID: 20439493 Free PMC article.
-
Shoc2-tranduced ERK1/2 motility signals--Novel insights from functional genomics.Cell Signal. 2016 May;28(5):448-459. doi: 10.1016/j.cellsig.2016.02.005. Epub 2016 Feb 11. Cell Signal. 2016. PMID: 26876614 Free PMC article.
References
-
- Arber, S., F. A. Barbayannis, H. Hanser, C. Schneider, C. A. Stanyon, O. Bernard, and P. Caroni. 1998. Regulation of actin dynamics through phosphorylation of cofilin by LIM-kinase. Nature 393:805-809. - PubMed
-
- Bobak, D., J. Moorman, A. Guanzon, L. Gilmer, and C. Hahn. 1997. Inactivation of the small GTPase Rho disrupts cellular attachment and induces adhesion-dependent and adhesion-independent apoptosis. Oncogene 15:2179-2189. - PubMed
-
- Bowman, A. B., R. S. Patel-King, S. E. Benashski, J. M. McCaffery, L. S. Goldstein, and S. M. King. 1999. Drosophila roadblock and Chlamydomonas LC7: a conserved family of dynein-associated proteins involved in axonal transport, flagellar motility, and mitosis. J. Cell Biol. 146:165-180. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous
