The erythroid phenotype of EKLF-null mice: defects in hemoglobin metabolism and membrane stability
- PMID: 15923635
- PMCID: PMC1140573
- DOI: 10.1128/MCB.25.12.5205-5214.2005
The erythroid phenotype of EKLF-null mice: defects in hemoglobin metabolism and membrane stability
Abstract
Development of red blood cells requires the correct regulation of cellular processes including changes in cell morphology, globin expression and heme synthesis. Transcription factors such as erythroid Kruppel-like factor EKLF (Klf1) play a critical role in erythropoiesis. Mice lacking EKLF die around embryonic day 14 because of defective definitive erythropoiesis, partly caused by a deficit in beta-globin expression. To identify additional target genes, we analyzed the phenotype and gene expression profiles of wild-type and EKLF null primary erythroid progenitors that were differentiated synchronously in vitro. We show that EKLF is dispensable for expansion of erythroid progenitors, but required for the last steps of erythroid differentiation. We identify EKLF-dependent genes involved in hemoglobin metabolism and membrane stability. Strikingly, expression of these genes is also EKLF-dependent in primitive, yolk sac-derived, blood cells. Consistent with lack of upregulation of these genes we find previously undetected morphological abnormalities in EKLF-null primitive cells. Our data provide an explanation for the hitherto unexplained severity of the EKLF null phenotype in erythropoiesis.
Figures
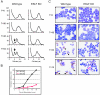
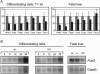
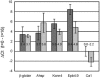
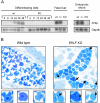


Similar articles
-
Erythroid Krüppel-like factor (EKLF) is active in primitive and definitive erythroid cells and is required for the function of 5'HS3 of the beta-globin locus control region.EMBO J. 1998 Apr 15;17(8):2334-41. doi: 10.1093/emboj/17.8.2334. EMBO J. 1998. PMID: 9545245 Free PMC article.
-
Erythroid Krüppel-like factor exhibits an early and sequentially localized pattern of expression during mammalian erythroid ontogeny.Dev Dyn. 1996 Jul;206(3):248-59. doi: 10.1002/(SICI)1097-0177(199607)206:3<248::AID-AJA3>3.0.CO;2-I. Dev Dyn. 1996. PMID: 8896981
-
Major erythrocyte membrane protein genes in EKLF-deficient mice.Exp Hematol. 2006 Jun;34(6):705-12. doi: 10.1016/j.exphem.2006.02.018. Exp Hematol. 2006. PMID: 16728274
-
Primitive erythropoiesis in the mammalian embryo.Int J Dev Biol. 2010;54(6-7):1011-8. doi: 10.1387/ijdb.093056jp. Int J Dev Biol. 2010. PMID: 20711979 Review.
-
Ontogeny of erythropoiesis.Curr Opin Hematol. 2008 May;15(3):155-61. doi: 10.1097/MOH.0b013e3282f97ae1. Curr Opin Hematol. 2008. PMID: 18391778 Review.
Cited by
-
Lineage-specific activators affect beta-globin locus chromatin in multipotent hematopoietic progenitors.EMBO J. 2006 Aug 9;25(15):3586-95. doi: 10.1038/sj.emboj.7601232. Epub 2006 Jul 13. EMBO J. 2006. PMID: 16858401 Free PMC article.
-
Failure of terminal erythroid differentiation in EKLF-deficient mice is associated with cell cycle perturbation and reduced expression of E2F2.Mol Cell Biol. 2008 Dec;28(24):7394-401. doi: 10.1128/MCB.01087-08. Epub 2008 Oct 13. Mol Cell Biol. 2008. PMID: 18852285 Free PMC article.
-
EKLF/KLF1 controls cell cycle entry via direct regulation of E2f2.J Biol Chem. 2009 Jul 31;284(31):20966-74. doi: 10.1074/jbc.M109.006346. Epub 2009 May 20. J Biol Chem. 2009. PMID: 19457859 Free PMC article.
-
Non-canonical hemoglobin: An updated review on its ubiquitous expression.Redox Biol. 2025 May;82:103602. doi: 10.1016/j.redox.2025.103602. Epub 2025 Mar 18. Redox Biol. 2025. PMID: 40138914 Free PMC article. Review.
-
A novel human cellular model of CDA IV enables comprehensive analysis revealing the molecular basis of the disease phenotype.Blood. 2023 Jun 22;141(25):3039-3054. doi: 10.1182/blood.2022018735. Blood. 2023. PMID: 37084386 Free PMC article.
References
-
- Bakker, W. J., M. Blazquez-Domingo, A. Kolbus, J. Besooyen, P. Steinlein, H. Beug, P. J. Coffer, B. Lowenberg, M. Von Lindern, and T. B. Van Dijk. 2004. FoxO3a regulates erythroid differentiation and induces BTG1, an activator of protein arginine methyl transferase 1. J. Cell Biol. 164:175-184. - PMC - PubMed
-
- Beug, H., S. Palmieri, C. Freudenstein, H. Zentgraf, and T. Graf. 1982. Hormone-dependent terminal differentiation in vitro of chicken erythroleukemia cells transformed by ts mutants of avian erythroblastosis virus. Cell 28:907-919. - PubMed
-
- Chen, J. J., and I. M. London. 1995. Regulation of protein synthesis by heme-regulated eIF-2 alpha kinase. Trends Biochem. Sci. 20:105-108. - PubMed
-
- Dolznig, H., F. Boulme, K. Stangl, E. M. Deiner, W. Mikulits, H. Beug, and E. W. Mullner. 2001. Establishment of normal, terminally differentiating mouse erythroid progenitors: molecular characterization by cDNA arrays. FASEB J. 15:1442-1444. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
