G1/S cell cycle arrest provides anoikis resistance through Erk-mediated Bim suppression
- PMID: 15923641
- PMCID: PMC1140593
- DOI: 10.1128/MCB.25.12.5282-5291.2005
G1/S cell cycle arrest provides anoikis resistance through Erk-mediated Bim suppression
Abstract
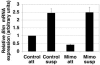
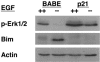
Proper attachment to the extracellular matrix is essential for cell survival. Detachment from the extracellular matrix results in an apoptotic process termed anoikis. Anoikis induction in MCF-10A mammary epithelial cells is due not only to loss of survival signals following integrin disengagement, but also to consequent downregulation of epidermal growth factor (EGFR) and loss of EGFR-induced survival signals. Here we demonstrate that G(1)/S arrest by overexpression of the cyclin-dependent kinase inhibitors p16(INK4a), p21(Cip1), or p27(Kip1) or by treatment with mimosine or aphidicolin confers anoikis resistance in MCF-10A cells. G(1)/S arrest-mediated anoikis resistance involves suppression of the BH3-only protein Bim. Furthermore, in G(1)/S-arrested cells, Erk phosphorylation is maintained in suspension and is necessary for Bim suppression. Following G(1)/S arrest, known proteins upstream of Erk, including Raf and Mek, are not activated. However, retained Erk activation under conditions in which Raf and Mek activation is lost is observed, suggesting that G(1)/S arrest acts at the level of Erk dephosphorylation. Thus, anoikis resistance by G(1)/S arrest is mediated by a mechanism involving Bim suppression through maintenance of Erk activation. These results provide a novel link between cell cycle arrest and survival, and this mechanism could contribute to the survival of nonreplicating, dormant tumor cells that avert apoptosis during early stages of metastasis.
Figures







References
-
- Allan, L. A., N. Morrice, S. Brady, G. Magee, S. Pathak, and P. R. Clarke. 2003. Inhibition of caspase-9 through phosphorylation at Thr 125 by ERK MAPK. Nat. Cell Biol. 5:647-654. - PubMed
-
- Alpan, R. S., and A. B. Pardee. 1996. p21WAF1/CIP1/SDI1 is elevated through a p53-independent pathway by mimosine. Cell Growth Differ. 7:893-901. - PubMed
-
- Biswas, S. C., and L. A. Greene. 2002. Nerve growth factor (NGF) down-regulates the Bcl-2 homology 3 (BH3) domain-only protein Bim and suppresses its proapoptotic activity by phosphorylation. J. Biol. Chem. 277:49511-49516. - PubMed
-
- Blagosklonny, M. V. 2002. Are p27 and p21 cytoplasmic oncoproteins? Cell Cycle 1:391-393. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
