Nonclassical, distinct endocytic signals dictate constitutive and PKC-regulated neurotransmitter transporter internalization
- PMID: 15924135
- PMCID: PMC2597780
- DOI: 10.1038/nn1478
Nonclassical, distinct endocytic signals dictate constitutive and PKC-regulated neurotransmitter transporter internalization
Abstract
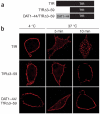
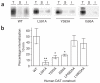
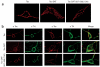
Neurotransmitter transporters are critical for synaptic neurotransmitter inactivation. Transporter inhibitors markedly increase the duration and magnitude of synaptic transmission, underscoring the importance of transporter activity in neurotransmission. Recent studies indicate that membrane trafficking dynamically governs neuronal transporter cell-surface presentation in a protein kinase C-regulated manner, suggesting that transporter trafficking profoundly affects synaptic signaling. However, the molecular architecture coupling neurotransmitter transporters to the endocytic machinery is not defined. Here, we identify nonclassical, distinct endocytic signals in the dopamine transporter (DAT) that are necessary and sufficient to drive constitutive and protein kinase C-regulated DAT internalization. The DAT internalization signal is conserved across SLC6 neurotransmitter carriers and is functional in the homologous norepinephrine transporter, suggesting that this region is likely to be the endocytic signal for all SLC6 neurotransmitter transporters. The DAT endocytic signal does not conform to classic internalization motifs, suggesting that SLC6 neurotransmitter transporters may have evolved unique endocytic mechanisms.
Figures



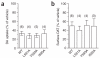

References
-
- Chen NH, Reith ME, Quick MW. Synaptic uptake and beyond: the sodium- and chloride-dependent neurotransmitter transporter family SLC6. Pflugers Arch. 2004;447:519–531. - PubMed
-
- Torres GE, Gainetdinov RR, Caron MG. Plasma membrane monoamine transporters: structure, regulation and function. Nat. Rev. Neurosci. 2003;4:13–25. - PubMed
-
- Rothman RB, Baumann MH. Monoamine transporters and psychostimulant drugs. Eur. J. Pharmacol. 2003;479:23–40. - PubMed
-
- Schenk JO. The functioning neuronal transporter for dopamine: kinetic mechanisms and effects of amphetamines, cocaine and methylphenidate. Prog. Drug Res. 2002;59:111–131. - PubMed
-
- Gulley JM, Zahniser NR. Rapid regulation of dopamine transporter function by substrates blockers and presynaptic receptor ligands. Eur. J. Pharmacol. 2003;479:139–152. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

