Stable T cell-dendritic cell interactions precede the development of both tolerance and immunity in vivo
- PMID: 15924144
- PMCID: PMC1560107
- DOI: 10.1038/ni1210
Stable T cell-dendritic cell interactions precede the development of both tolerance and immunity in vivo
Abstract
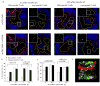
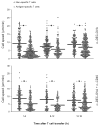
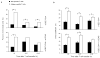
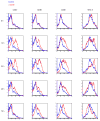
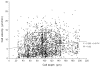
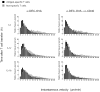
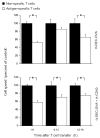
The maturation status of dendritic cells (DCs) determines whether they prime or tolerize T cells. We targeted ovalbumin peptide exclusively to DCs in situ using an antibody to DEC-205 and studied the interaction of DCs with naive CD4(+) T cells in tolerizing or priming conditions. We used two-photon microscopy to simultaneously track antigen-specific OT-II T cells, nonspecific T cells and DCs in lymph nodes of living mice. In both tolerance and immunity, OT-II cells arrested on DCs near high endothelial venules beginning shortly after extravasation and regained their baseline speed by 18 h. Thus, early antigen-dependent T cell arrest on DCs is a shared feature of tolerance and priming associated with activation and proliferation.
Conflict of interest statement
Figures




References
-
- Stoll S, Delon J, Brotz TM, Germain RN. Dynamic imaging of T cell-dendritic cell interactions in lymph nodes. Science. 2002;296:1873–6. - PubMed
-
- Miller MJ, Wei SH, Parker I, Cahalan MD. Two-Photon Imaging of Lymphocyte Motility and Antigen Response in Intact Lymph Node. Science. 2002;296:1869–1873. - PubMed
-
- Bousso P, Robey E. Dynamics of CD8+ T cell priming by dendritic cells in intact lymph nodes. Nat Immunol. 2003;4:579–85. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
