Serum neopterin for early assessment of severity of severe acute respiratory syndrome
- PMID: 15925828
- PMCID: PMC7106326
- DOI: 10.1016/j.clim.2005.03.009
Serum neopterin for early assessment of severity of severe acute respiratory syndrome
Abstract
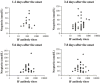
Neopterin and C-reactive protein (CRP) concentrations were determined in serum samples from 129 severe acute respiratory syndrome (SARS) patients and 156 healthy blood donors. In the patients with confirmed SARS, an early neopterin elevation was detected already at the day of onset of symptoms and rose to a maximum level of 45.0 nmol/L 3 days after the onset. All SARS patients had elevated neopterin concentrations (>10 nmol/L) within 9 days after the onset. The mean neopterin concentrations were 34.2 nmol/L in acute sera of SARS patients, 5.1 nmol/L in convalescent sera, and 6.7 nmol/L in healthy controls. In contrast, the mean CRP concentrations in both acute and convalescent sera of SARS patients were in the normal range (<10 mg/L). Serum neopterin level in SARS patients was associated with fever period and thus the clinical progression of the disease, while there was no significant correlation between the CRP level and the fever period. Serum neopterin may allow early assessment of the severity of SARS. The decrease of neopterin level was found after steroid treatment, which indicates that blood samples should be collected before steroid treatment for the neopterin measurement.
Figures




References
-
- Centers for Disease Control and Prevention . 2003. Severe Acute Respiratory Syndrome (SARS) Fact Sheet. http://www.cdc.gov/ncidod/sars/pdf/factsheet.pdf.
-
- Rota P.A., Oberste M.S., Monroe S.S., Nix W.A., Campagnoli R., Icenogle J.P. Characterization of a novel coronavirus associated with severe acute respiratory syndrome. Science. 2003;300:1394–1399. - PubMed
-
- Marra M.A., Jones S.J., Astell C.R., Holt R.A., Brooks-Wilson A., Butterfield Y.S. The genome sequence of the SARS-associated coronavirus. Science. 2003;300:1399–1404. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

