Brown recluse spider (Loxosceles reclusa) venom phospholipase D (PLD) generates lysophosphatidic acid (LPA)
- PMID: 15926888
- PMCID: PMC1276930
- DOI: 10.1042/BJ20050043
Brown recluse spider (Loxosceles reclusa) venom phospholipase D (PLD) generates lysophosphatidic acid (LPA)
Abstract
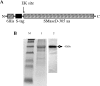
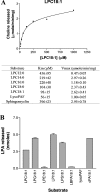
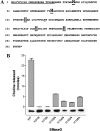
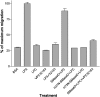
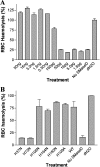
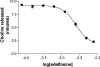
Envenomation by the brown recluse spider (Loxosceles reclusa) may cause local dermonecrosis and, rarely, coagulopathies, kidney failure and death. A venom phospholipase, SMaseD (sphingomyelinase D), is responsible for the pathological manifestations of envenomation. Recently, the recombinant SMaseD from Loxosceles laeta was demonstrated to hydrolyse LPC (lysophosphatidylcholine) to produce LPA (lysophosphatidic acid) and choline. Therefore activation of LPA signalling pathways may be involved in some manifestations of Loxosceles envenomation. To begin investigating this idea, we cloned a full-length cDNA encoding L. reclusa SMaseD. The 305 amino acid sequence of the L. reclusa enzyme is 87, 85 and 60% identical with those of L. arizonica, L. intermedia and L. laeta respectively. The recombinant enzyme expressed in bacteria had broad substrate specificity. The lysophospholipids LPC, LPI (18:1-1-oleyol lysophosphatidylinositol), LPS, LPG (18:1-1-oleoyl-lysophosphatidylglycerol), LBPA (18:1-1-oleoyl-lysobisphosphatidic acid) (all with various acyl chains), lyso-platelet-activating factor (C16:0), cyclic phosphatidic acid and sphingomyelin were hydrolysed, whereas sphingosylphosphorylcholine, PC (phosphatidylcholine; C22:6, C20:4 and C6:0), oxidized PCs and PAF (platelet-activating factor; C16:0) were not hydrolysed. The PAF analogue, edelfosine, inhibited enzyme activity. Recombinant enzyme plus LPC (C18:1) induced the migration of A2058 melanoma cells, and this activity was blocked by the LPA receptor antagonist, VPC32183. The recombinant spider enzyme was haemolytic, but this activity was absent from catalytically inactive H37N (His37-->Asn) and H73N mutants. Our results demonstrate that Loxosceles phospholipase D hydrolyses a wider range of lysophospholipids than previously supposed, and thus the term 'SMaseD' is too limited in describing this enzyme.
Figures


Similar articles
-
Sphingomyelinase D from venoms of Loxosceles spiders: evolutionary insights from cDNA sequences and gene structure.Toxicon. 2005 Apr;45(5):547-60. doi: 10.1016/j.toxicon.2004.11.011. Toxicon. 2005. PMID: 15777950
-
Modulation of membrane phospholipids, the cytosolic calcium influx and cell proliferation following treatment of B16-F10 cells with recombinant phospholipase-D from Loxosceles intermedia (brown spider) venom.Toxicon. 2013 Jun 1;67:17-30. doi: 10.1016/j.toxicon.2013.01.027. Epub 2013 Feb 24. Toxicon. 2013. PMID: 23462381
-
A Brief Overview of the Toxic Sphingomyelinase Ds of Brown Recluse Spider Venom and Other Organisms and Simple Methods To Detect Production of Its Signature Cyclic Ceramide Phosphate.Mol Pharmacol. 2024 Feb 15;105(3):144-154. doi: 10.1124/molpharm.123.000709. Mol Pharmacol. 2024. PMID: 37739813 Free PMC article.
-
Brown recluse spider (Loxosceles reclusa) envenomation in small animals.J Vet Emerg Crit Care (San Antonio). 2009 Aug;19(4):329-36. doi: 10.1111/j.1476-4431.2009.00440.x. Epub 2009 Jul 28. J Vet Emerg Crit Care (San Antonio). 2009. PMID: 25164631 Review.
-
Arachnids submitted as suspected brown recluse spiders (Araneae: Sicariidae): Loxosceles spiders are virtually restricted to their known distributions but are perceived to exist throughout the United States.J Med Entomol. 2005 Jul;42(4):512-21. doi: 10.1093/jmedent/42.4.512. J Med Entomol. 2005. PMID: 16119538 Review.
Cited by
-
Sphingomyelinase D activity in model membranes: structural effects of in situ generation of ceramide-1-phosphate.PLoS One. 2012;7(4):e36003. doi: 10.1371/journal.pone.0036003. Epub 2012 Apr 25. PLoS One. 2012. PMID: 22558302 Free PMC article.
-
Exploring the Venom Gland Transcriptome of Bothrops asper and Bothrops jararaca: De Novo Assembly and Analysis of Novel Toxic Proteins.Toxins (Basel). 2024 Nov 27;16(12):511. doi: 10.3390/toxins16120511. Toxins (Basel). 2024. PMID: 39728769 Free PMC article.
-
The critical roles of bioactive sphingolipids in inflammation.J Biol Chem. 2025 Aug;301(8):110475. doi: 10.1016/j.jbc.2025.110475. Epub 2025 Jul 11. J Biol Chem. 2025. PMID: 40653199 Free PMC article. Review.
-
Brown Spider (Loxosceles) Venom Toxins as Potential Biotools for the Development of Novel Therapeutics.Toxins (Basel). 2019 Jun 19;11(6):355. doi: 10.3390/toxins11060355. Toxins (Basel). 2019. PMID: 31248109 Free PMC article. Review.
-
Variable Substrate Preference among Phospholipase D Toxins from Sicariid Spiders.J Biol Chem. 2015 Apr 24;290(17):10994-1007. doi: 10.1074/jbc.M115.636951. Epub 2015 Mar 9. J Biol Chem. 2015. PMID: 25752604 Free PMC article.
References
-
- Sams H. H., Dunnick C. A., Smith M. L., King L. E., Jr Necrotic arachnidism. J. Am. Acad. Dermatol. 2001;44:561–573. - PubMed
-
- Tambourgi D. V., Magnoli F. C., van den Berg C. W., Morgan B. P., de Araujo P. S., Alves E. W., Da Silva W. D. Sphingomyelinases in the venom of the spider Loxosceles intermedia are responsible for both dermonecrosis and complement-dependent hemolysis. Biochem. Biophys. Res. Commun. 1998;251:366–373. - PubMed
-
- Zanetti V. C., da Silveira R. B., Dreyfuss J. L., Haoach J., Mangili O. C., Veiga S. S., Gremski W. Morphological and biochemical evidence of blood vessel damage and fibrinogenolysis triggered by brown spider venom. Blood Coagul. Fibrinolysis. 2002;13:135–148. - PubMed
-
- Gulbins E., Kolesnick R. Raft ceramide in molecular medicine. Oncogene. 2003;22:7070–7077. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

