Human ADA2 belongs to a new family of growth factors with adenosine deaminase activity
- PMID: 15926889
- PMCID: PMC1237138
- DOI: 10.1042/BJ20050683
Human ADA2 belongs to a new family of growth factors with adenosine deaminase activity
Abstract
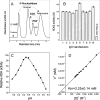
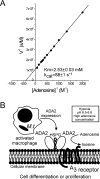
Two distinct isoenzymes of ADA (adenosine deaminase), ADA1 and ADA2, have been found in humans. Inherited mutations in ADA1 result in SCID (severe combined immunodeficiency). This observation has led to extensive studies of the structure and function of this enzyme that have revealed an important role for it in lymphocyte activation. In contrast, the physiological role of ADA2 is unknown. ADA2 is found in negligible quantities in serum and may be produced by monocytes/macrophages. ADA2 activity in the serum is increased in various diseases in which monocyte/macrophage cells are activated. In the present study, we report that ADA2 is a heparin-binding protein. This allowed us to obtain a highly purified enzyme and to study its biochemistry. ADA2 was identified as a member of a new class of ADGFs (ADA-related growth factors), which is present in almost all organisms from flies to humans. Our results suggest that ADA2 may be active in sites of inflammation during hypoxia and in areas of tumour growth where the adenosine concentration is significantly elevated and the extracellular pH is acidic. Our finding that ADA2 co-purified and concentrated together with IgG in commercially available preparations offers an intriguing explanation for the observation that treatment with such preparations leads to non-specific immune-system stimulation.
Figures


Similar articles
-
Structural basis for the growth factor activity of human adenosine deaminase ADA2.J Biol Chem. 2010 Apr 16;285(16):12367-77. doi: 10.1074/jbc.M109.083527. Epub 2010 Feb 9. J Biol Chem. 2010. PMID: 20147294 Free PMC article.
-
Adenosine deaminase-related genes: molecular identification, tissue expression pattern and truncated alternative splice isoform in adult zebrafish (Danio rerio).Life Sci. 2007 Nov 10;81(21-22):1526-34. doi: 10.1016/j.lfs.2007.09.019. Epub 2007 Oct 4. Life Sci. 2007. PMID: 17950365
-
Human adenosine deaminase 2 induces differentiation of monocytes into macrophages and stimulates proliferation of T helper cells and macrophages.J Leukoc Biol. 2010 Aug;88(2):279-90. doi: 10.1189/jlb.1109764. Epub 2010 May 7. J Leukoc Biol. 2010. PMID: 20453107
-
[Adenosine deaminase: isoenzymes ADA1 and ADA2].Pol Merkur Lekarski. 1997 Dec;3(18):288-90. Pol Merkur Lekarski. 1997. PMID: 9523470 Review. Polish.
-
[Adenosine deaminase].Nihon Rinsho. 1995 May;53(5):1178-83. Nihon Rinsho. 1995. PMID: 7602776 Review. Japanese.
Cited by
-
Therapeutic Perspectives of Adenosine Deaminase Inhibition in Cardiovascular Diseases.Molecules. 2020 Oct 12;25(20):4652. doi: 10.3390/molecules25204652. Molecules. 2020. PMID: 33053898 Free PMC article. Review.
-
Getting to know adenosine deaminase 2 deficiency inside and out.J Allergy Clin Immunol. 2025 May;155(5):1451-1463. doi: 10.1016/j.jaci.2025.01.040. Epub 2025 Feb 14. J Allergy Clin Immunol. 2025. PMID: 39956283 Free PMC article. Review.
-
Pathogenic variant c.1052T>A (p.Leu351Gln) in adenosine deaminase 2 impairs secretion and elevates type I IFN responsive gene expression.Front Immunol. 2022 Sep 30;13:995191. doi: 10.3389/fimmu.2022.995191. eCollection 2022. Front Immunol. 2022. PMID: 36248868 Free PMC article.
-
Vasculitis and vasculopathy associated with inborn errors of immunity: an overview.Front Pediatr. 2024 Jan 31;11:1258301. doi: 10.3389/fped.2023.1258301. eCollection 2023. Front Pediatr. 2024. PMID: 38357265 Free PMC article. Review.
-
Polyarteritis nodosa and deficiency of adenosine deaminase 2 - Shared genealogy, generations apart.Clin Immunol. 2020 Jun;215:108411. doi: 10.1016/j.clim.2020.108411. Epub 2020 Apr 7. Clin Immunol. 2020. PMID: 32276138 Free PMC article. Review.
References
-
- Ratech H., Thorbecke G. J., Meredith G., Hirschhorn R. Comparison and possible homology of isozymes of adenosine deaminase in Aves and humans. Enzyme. 1981;26:74–84. - PubMed
-
- Schrader W. P., Woodward F. J., Pollara B. Purification of an adenosine deaminase complexing protein from human plasma. J. Biol. Chem. 1979;254:11964–11968. - PubMed
-
- Weihofen W. A., Liu J., Reutter W., Saenger W., Fan H. Crystal structure of CD26/dipeptidyl-peptidase IV in complex with adenosine deaminase reveals a highly amphiphilic interface. J. Biol. Chem. 2004;279:43330–43335. - PubMed
-
- Ungerer J. P., Oosthuizen H. M., Bissbort S. H., Vermaak W. J. Serum adenosine deaminase: isoenzymes and diagnostic application. Clin. Chem. 1992;38:1322–1326. - PubMed
-
- Thompson L. F., Seegmiller J. E. Adenosine deaminase deficiency and severe combined immunodeficiency disease. Adv. Enzymol. Relat. Areas Mol. Biol. 1980;51:167–210. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

