N-glycans of core2 beta(1,6)-N-acetylglucosaminyltransferase-I (C2GnT-I) but not those of alpha(1,3)-fucosyltransferase-VII (FucT-VII) are required for the synthesis of functional P-selectin glycoprotein ligand-1 (PSGL-1): effects on P-, L- and E-selectin binding
- PMID: 15926890
- PMCID: PMC1276950
- DOI: 10.1042/BJ20050344
N-glycans of core2 beta(1,6)-N-acetylglucosaminyltransferase-I (C2GnT-I) but not those of alpha(1,3)-fucosyltransferase-VII (FucT-VII) are required for the synthesis of functional P-selectin glycoprotein ligand-1 (PSGL-1): effects on P-, L- and E-selectin binding
Abstract
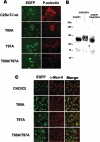
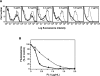
C2GnT-I [core2 beta(1,6)-N-acetyglucosaminyltransferase-I] and FucT-VII [alpha(1,3)-fucosyltransferase-VII] are the key enzymes for the biosynthesis of sialyl-Lewis x determinants on selectin ligands and therefore they represent good drug targets for the treatment of inflammatory disorders and other pathologies involving selectins. In the present study, we examined the importance of N-glycosylation for the ability of C2GnT-I and FucT-VII to generate functional selectin ligands, particularly the PSGL-1 (P-selectin glycoprotein ligand-1). We found that (i) both enzymes have their two N-glycosylation sites occupied, (ii) for C2GnT-I, the N-glycan chain linked to Asn-95 significantly contributes to the synthesis of functional PSGL-1 and is required to localize the enzyme to the cis/medial-Golgi compartment, (iii) all N-glycosylation-deficient proteins of FucT-VII displayr a dramatic impairment of their in vitro enzymatic activities, but retain their ability to fucosylate the core2-modified PSGL-I and to generate P- and L-selectin binding, and (iv) the glycomutants of FucT-VII fail to synthesize sialyl-Lewis x or to generate E-selectin binding unless core2-modified PSGL-1 is present. All combined, our results show a differential functional impact of N-glycosylation on C2GnT-1 and FucT-VII and disclose that a strongly reduced FucT-VII activity retains the ability to fucosylate PSGL-1 on the core2-based binding site(s) for the three selectins.
Figures






References
-
- Springer T. A. Traffic signals for lymphocyte recirculation and leukocyte emigration: the multistep paradigm. Cell (Cambridge, Mass.) 1994;76:301–331. - PubMed
-
- Maksimowicz-McKinnon K., Bhatt D. L., Calabrese L. H. Recent advances in vascular inflammation: C-reactive protein and other inflammatory biomarkers. Curr. Opin. Rheumatol. 2004;16:18–24. - PubMed
-
- Lowe J. B. Glycan-dependent leukocyte adhesion and recruitment in inflammation. Curr. Opin. Cell Biol. 2003;15:531–538. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

