Development of occlusive neointimal lesions in distal pulmonary arteries of endothelin B receptor-deficient rats: a new model of severe pulmonary arterial hypertension
- PMID: 15927975
- PMCID: PMC1934986
- DOI: 10.1161/CIRCULATIONAHA.104.491456
Development of occlusive neointimal lesions in distal pulmonary arteries of endothelin B receptor-deficient rats: a new model of severe pulmonary arterial hypertension
Abstract
Background: Human pulmonary arterial hypertension (PAH) is characterized by proliferation of vascular smooth muscle and, in its more severe form, by the development of occlusive neointimal lesions. However, few animal models of pulmonary neointimal proliferation exist, thereby limiting a complete understanding of the pathobiology of PAH. Recent studies of the endothelin (ET) system demonstrate that deficiency of the ET(B) receptor predisposes adult rats to acute and chronic hypoxic PAH, yet these animals fail to develop neointimal lesions. Herein, we determined and thereafter showed that exposure of ET(B) receptor-deficient rats to the endothelial toxin monocrotaline (MCT) leads to the development of neointimal lesions that share hallmarks of human PAH.
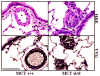
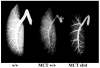
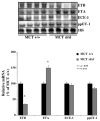
Methods and results: The pulmonary hemodynamic and morphometric effects of 60 mg/kg MCT in control (MCT(+/+)) and ET(B) receptor-deficient (MCT(sl/sl)) rats at 6 weeks of age were assessed. MCT(sl/sl) rats developed more severe PAH, characterized by elevated pulmonary artery pressure, diminished cardiac output, and right ventricular hypertrophy. In MCT(sl/sl) rats, morphometric evaluation revealed the presence of neointimal lesions within small distal pulmonary arteries, increased medial wall thickness, and decreased arterial-to-alveolar ratio. In keeping with this, barium angiography revealed diminished distal pulmonary vasculature of MCT(sl/sl) rat lungs. Cells within neointimal lesions expressed smooth muscle and endothelial cell markers. Moreover, cells within neointimal lesions exhibited increased levels of proliferation and were located in a tissue microenvironment enriched with vascular endothelial growth factor, tenascin-C, and activated matrix metalloproteinase-9, factors already implicated in human PAH. Finally, assessment of steady state mRNA showed that whereas expression of ET(B) receptors was decreased in MCT(sl/sl) rat lungs, ET(A) receptor expression increased.
Conclusions: Deficiency of the ET(B) receptor markedly accelerates the progression of PAH in rats treated with MCT and enhances the appearance of cellular and molecular markers associated with the pathobiology of PAH. Collectively, these results suggest an overall antiproliferative effect of the ET(B) receptor in pulmonary vascular homeostasis.
Figures




Similar articles
-
Simvastatin rescues rats from fatal pulmonary hypertension by inducing apoptosis of neointimal smooth muscle cells.Circulation. 2003 Sep 30;108(13):1640-5. doi: 10.1161/01.CIR.0000087592.47401.37. Epub 2003 Sep 8. Circulation. 2003. PMID: 12963647
-
Pulmonary hemodynamics modify the rat pulmonary artery response to injury. A neointimal model of pulmonary hypertension.Am J Pathol. 1997 Oct;151(4):1019-25. Am J Pathol. 1997. PMID: 9327735 Free PMC article.
-
[Mechanism of vitamin D deficiency involvement in the development of pulmonary arterial hypertension related to monocrotaline-induced connective tissue disease in rats].Zhonghua Yi Xue Za Zhi. 2024 Nov 19;104(43):3972-3979. doi: 10.3760/cma.j.cn112137-20240401-00742. Zhonghua Yi Xue Za Zhi. 2024. PMID: 39533688 Chinese.
-
The role of disturbed blood flow in the development of pulmonary arterial hypertension: lessons from preclinical animal models.Am J Physiol Lung Cell Mol Physiol. 2013 Jul 1;305(1):L1-14. doi: 10.1152/ajplung.00031.2013. Epub 2013 Apr 26. Am J Physiol Lung Cell Mol Physiol. 2013. PMID: 23624788 Review.
-
[Animal models of pulmonary arterial hypertension].Rev Mal Respir. 2007 Apr;24(4 Pt 1):481-96. doi: 10.1016/s0761-8425(07)91571-5. Rev Mal Respir. 2007. PMID: 17468705 Review. French.
Cited by
-
Idiopathic pulmonary arterial hypertension.Dis Model Mech. 2010 May-Jun;3(5-6):268-73. doi: 10.1242/dmm.003616. Dis Model Mech. 2010. PMID: 20427556 Free PMC article. Review.
-
The xanthine derivative KMUP-1 inhibits models of pulmonary artery hypertension via increased NO and cGMP-dependent inhibition of RhoA/Rho kinase.Br J Pharmacol. 2010 Jun;160(4):971-86. doi: 10.1111/j.1476-5381.2010.00740.x. Br J Pharmacol. 2010. PMID: 20590592 Free PMC article.
-
Arterial wall stress controls NFAT5 activity in vascular smooth muscle cells.J Am Heart Assoc. 2014 Mar 10;3(2):e000626. doi: 10.1161/JAHA.113.000626. J Am Heart Assoc. 2014. PMID: 24614757 Free PMC article.
-
A current view of G protein-coupled receptor - mediated signaling in pulmonary hypertension: finding opportunities for therapeutic intervention.Vessel Plus. 2018;2:29. doi: 10.20517/2574-1209.2018.44. Epub 2018 Aug 30. Vessel Plus. 2018. PMID: 31380505 Free PMC article.
-
Hemodynamic and histologic characterization of a swine (Sus scrofa domestica) model of chronic pulmonary arterial hypertension.Comp Med. 2011 Jun;61(3):258-62. Comp Med. 2011. PMID: 21819696 Free PMC article.
References
-
- Rabinovitch M, Haworth SG, Castaneda AR, Nadas AS, Reid LM. Lung biopsy in congenital heart disease: a morphometric approach to pulmonary vascular disease. Circulation. 1978;58:1107–1122. - PubMed
-
- Rabinovitch M. Pathobiology of pulmonary hypertension: extracellular matrix. Clin Chest Med. 2001;22:433–449. - PubMed
-
- Tuder RM, Chacon M, Alger L, Wang J, Taraseviciene-Stewart L, Kasahara Y, Cool CD, Bishop AE, Geraci M, Semenza GL, Yacoub M, Polak JM, Voelkel NF. Expression of angiogenesis-related molecules in plexiform lesions in severe pulmonary hypertension: evidence for a process of disordered angiogenesis. J Pathol. 2001;195:367–374. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

