The target of ezetimibe is Niemann-Pick C1-Like 1 (NPC1L1)
- PMID: 15928087
- PMCID: PMC1149415
- DOI: 10.1073/pnas.0500269102
The target of ezetimibe is Niemann-Pick C1-Like 1 (NPC1L1)
Abstract
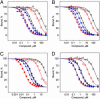
Ezetimibe is a potent inhibitor of cholesterol absorption that has been approved for the treatment of hypercholesterolemia, but its molecular target has been elusive. Using a genetic approach, we recently identified Niemann-Pick C1-Like 1 (NPC1L1) as a critical mediator of cholesterol absorption and an essential component of the ezetimibe-sensitive pathway. To determine whether NPC1L1 is the direct molecular target of ezetimibe, we have developed a binding assay and shown that labeled ezetimibe glucuronide binds specifically to a single site in brush border membranes and to human embryonic kidney 293 cells expressing NPC1L1. Moreover, the binding affinities of ezetimibe and several key analogs to recombinant NPC1L1 are virtually identical to those observed for native enterocyte membranes. KD values of ezetimibe glucuronide for mouse, rat, rhesus monkey, and human NPC1L1 are 12,000, 540, 40, and 220 nM, respectively. Last, ezetimibe no longer binds to membranes from NPC1L1 knockout mice. These results unequivocally establish NPC1L1 as the direct target of ezetimibe and should facilitate efforts to identify the molecular mechanism of cholesterol transport.
Figures




References
-
- Grundy, S. M. (1983) Annu. Rev. Nutr. 3, 71-96. - PubMed
-
- Clader, J. W. (2004) J. Med. Chem. 47, 1-9. - PubMed
-
- Davis, H. R., Jr., Zhu, L. J., Hoos, L. M., Tetzloff, G., Maguire, M., Liu, J., Yao, X., Iyer, S. P., Lam, M. H., Lund, E. G., et al. (2004) J. Biol. Chem. 279, 33586-33592. - PubMed
-
- Bays, H. E., Moore, P. B., Drehobl, M. A., Rosenblatt, S., Toth, P. D., Dujovne, C. A., Knopp, R. H., Lipka, L. J., Lebeaut, A. P., Yang, B., et al. (2001) Clin. Ther. 23, 1209-1230. - PubMed
-
- Dujovne, C. A., Ettinger, M. P., McNeer, J. F., Lipka, L. J., LeBeaut, A. P., Suresh, R., Yang, B. & Veltri, E. P. (2002) Am. J. Cardiol. 90, 1092-1097. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

