The pseudouridine synthase RluD is required for normal ribosome assembly and function in Escherichia coli
- PMID: 15928344
- PMCID: PMC1370798
- DOI: 10.1261/rna.2550105
The pseudouridine synthase RluD is required for normal ribosome assembly and function in Escherichia coli
Abstract
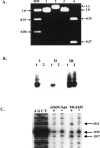
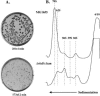
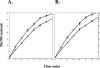
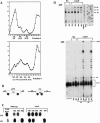
RluD is the pseudouridine synthase responsible for the formation of Psi1911, Psi1915, and Psi1917 in Escherichia coli 23S rRNA. Previous work from our laboratory demonstrated that disruption of the rluD gene and/or loss of the pseudouridine residues for which it is responsible resulted in a severe growth phenotype. In the current work we have examined further the effect of the loss of the RluD protein and its product pseudouridine residues in a deletion strain lacking the rluD gene. This strain exhibits defects in ribosome assembly, biogenesis, and function. Specifically, there is a deficit of 70S ribosomes, an increase in 50S and 30S subunits, and the appearance of new 62S and 39S particles. Analysis of the 39S particles indicates that they are immature precursors of the 50S subunits, whereas the 62S particles are derived from the breakdown of unstable 70S ribosomes. In addition, purified mutant 70S ribosomes were found to be somewhat less efficient than wild type in protein synthesis. The defect in ribosome assembly and resulting growth phenotype of the mutant could be restored by expression of wild-type RluD and synthesis of Psi1911, Psi1915, and Psi1917 residues, but not by catalytically inactive mutant RluD proteins, incapable of pseudouridine formation. The data suggest that the loss of the pseudouridine residues can account for all aspects of the mutant phenotype; however, a possible second function of the RluD synthase is also discussed.
Figures

References
-
- Bashan, A., Agmon, I., Zarivach, R., Schluenzen, F., Harms, J., Berisio, R., Bartels, H., Franceschi, F., Auerach, T., Hansen, H.A.S., et al. 2003. Structural basis of the ribosomal machinery for peptide bond formation, translocation, and nascent chain progression. Mol. Cell 11: 91–102. - PubMed
-
- Behm-Ansmant, I., Grosjean, H., Massenet, S., and Motorin, Y. 2004. Pseudouridylation at position 32 of mitochondrial and cytoplasmic tRNAs requires two distinct enzymes in Saccharomyces cerevisiae. J. Biol. Chem. 279: 52998–53006. - PubMed
-
- Charollais, J., Pflieger, D., Vinh, J., Dreyfus, M., and Iost, I. 2003. The DEAD-box RNA helicase SrmB is involved in the assembly of 50S ribosomal subunits in Escherichia coli. Mol. Microbiol. 48: 1253–1265. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
