The frequency of calcium oscillations induced by 5-HT, ACH, and KCl determine the contraction of smooth muscle cells of intrapulmonary bronchioles
- PMID: 15928401
- PMCID: PMC2234076
- DOI: 10.1085/jgp.200409216
The frequency of calcium oscillations induced by 5-HT, ACH, and KCl determine the contraction of smooth muscle cells of intrapulmonary bronchioles
Abstract
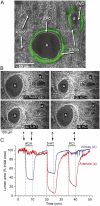
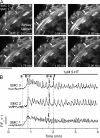
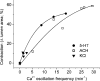
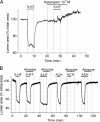
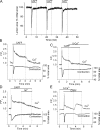
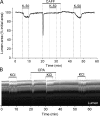
Increased resistance of airways or blood vessels within the lung is associated with asthma or pulmonary hypertension and results from contraction of smooth muscle cells (SMCs). To study the mechanisms regulating these contractions, we developed a mouse lung slice preparation containing bronchioles and arterioles and used phase-contrast and confocal microscopy to correlate the contractile responses with changes in [Ca(2+)](i) of the SMCs. The airways are the focus of this study. The agonists, 5-hydroxytrypamine (5-HT) and acetylcholine (ACH) induced a concentration-dependent contraction of the airways. High concentrations of KCl induced twitching of the airway SMCs but had little effect on airway size. 5-HT and ACH induced asynchronous oscillations in [Ca(2+)](i) that propagated as Ca(2+) waves within the airway SMCs. The frequency of the Ca(2+) oscillations was dependent on the agonist concentration and correlated with the extent of sustained airway contraction. In the absence of extracellular Ca(2+) or in the presence of Ni(2+), the frequency of the Ca(2+) oscillations declined and the airway relaxed. By contrast, KCl induced low frequency Ca(2+) oscillations that were associated with SMC twitching. Each KCl-induced Ca(2+) oscillation consisted of a large Ca(2+) wave that was preceded by multiple localized Ca(2+) transients. KCl-induced responses were resistant to neurotransmitter blockers but were abolished by Ni(2+) or nifedipine and the absence of extracellular Ca(2+). Caffeine abolished the contractile effects of 5-HT, ACH, and KCl. These results indicate that (a) 5-HT and ACH induce airway SMC contraction by initiating Ca(2+) oscillations, (b) KCl induces Ca(2+) transients and twitching by overloading and releasing Ca(2+) from intracellular stores, (c) a sustained, Ni(2+)-sensitive, influx of Ca(2+) mediates the refilling of stores to maintain Ca(2+) oscillations and, in turn, SMC contraction, and (d) the magnitude of sustained airway SMC contraction is regulated by the frequency of Ca(2+) oscillations.
Figures







Comment in
-
Imaging dynamic cellular events in the lung.J Gen Physiol. 2005 Jun;125(6):529-30. doi: 10.1085/jgp.200509322. J Gen Physiol. 2005. PMID: 15928399 Free PMC article. No abstract available.
References
-
- Abdullah, N.A., M. Hirata, K. Matsumoto, H. Aizawa, R. Inoue, S. Hamano, S. Ikeda, Z. Xie, N. Hara, and Y. Ito. 1994. Contraction and depolarization induced by fetal bovine serum in airway smooth muscle. Am. J. Physiol. 266:L528–L535. - PubMed
-
- Adler, A., E.A. Cowley, J.H. Bates, and D.H. Eidelman. 1998. Airway-parenchymal interdependence after airway contraction in rat lung explants. J. Appl. Physiol. 85:231–237. - PubMed
-
- Adriaensen, D., I. Brouns, J. Van Genechten, and J.P. Timmermans. 2003. Functional morphology of pulmonary neuroepithelial bodies: extremely complex airway receptors. Anat. Rec. A Discov. Mol. Cell Evol. Biol. 270:25–40. - PubMed
-
- Ay, B., Y.S. Prakash, C.M. Pabelick, and G.C. Sieck. 2004. Store-operated Ca2+ entry in porcine airway smooth muscle. Am. J. Physiol. Lung Cell. Mol. Physiol. 286:L909–L917. - PubMed
-
- Ben-Harari, R.R., A. Parent Ermini, and J. Kleinerman. 1990. Metabolism of 5-hydroxytryptophan in the isolated perfused rat lung. Pharmacology. 41:272–279. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

