Ethanol modulates the VR-1 variant amiloride-insensitive salt taste receptor. II. Effect on chorda tympani salt responses
- PMID: 15928404
- PMCID: PMC2234074
- DOI: 10.1085/jgp.200509264
Ethanol modulates the VR-1 variant amiloride-insensitive salt taste receptor. II. Effect on chorda tympani salt responses
Abstract
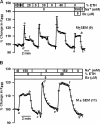
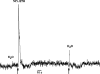
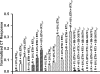
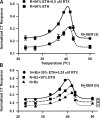
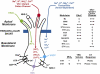
The effect of ethanol on the amiloride- and benzamil (Bz)-insensitive salt taste receptor was investigated by direct measurement of intracellular Na(+) activity ([Na(+)](i)) using fluorescence imaging in polarized fungiform taste receptor cells (TRCs) and by chorda tympani (CT) taste nerve recordings. CT responses to KCl and NaCl were recorded in Sprague-Dawley rats, and in wild-type (WT) and vanilloid receptor-1 (VR-1) knockout mice (KO). CT responses were monitored in the presence of Bz, a specific blocker of the epithelial Na(+) channel (ENaC). CT responses were also recorded in the presence of agonists (resiniferatoxin and elevated temperature) and antagonists (capsazepine and SB-366791) of VR-1 that similarly modulate the Bz-insensitive VR-1 variant salt taste receptor. In the absence of mineral salts, ethanol induced a transient decrease in TRC volume and elicited only transient phasic CT responses. In the presence of mineral salts, ethanol increased the apical cation flux in TRCs without a change in volume, increased transepithelial electrical resistance across the tongue, and elicited CT responses that were similar to salt responses, consisting of both a phasic component and a sustained tonic component. At concentrations <50%, ethanol enhanced responses to KCl and NaCl, while at ethanol concentrations >50%, those CT responses were inhibited. Resiniferatoxin and elevated temperature increased the sensitivity of the CT response to ethanol in salt-containing media, and SB-366791 inhibited the effect of ethanol, resiniferatoxin, and elevated temperature on the CT responses to mineral salts. VR-1 KO mice demonstrated no Bz-insensitive CT response to NaCl and no sensitivity to ethanol. We conclude that ethanol increases salt taste sensitivity by its direct action on the Bz-insensitive VR-1 variant salt taste receptor.
Figures




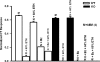
Comment in
-
The salty and burning taste of capsaicin.J Gen Physiol. 2005 Jun;125(6):531-4. doi: 10.1085/jgp.200509329. J Gen Physiol. 2005. PMID: 15928400 Free PMC article. No abstract available.
References
-
- Caterina, M.J., A. Leffler, A.B. Malmberg, W.J. Martin, J. Trafton, K.R. Petersen-Zeitz, M. Koltzenburg, A.I. Basbaum, and D. Julius. 2000. Impaired nociception and pain sensation in mice lacking the capsaicin receptor. Science. 288:306–313. - PubMed
-
- Danilova, V., and G. Hellekant. 2000. The taste of ethanol in a primate model. II. Glossopharyngeal nerve response in Macaca mulatta. Alcohol. 21:259–269. - PubMed
-
- Davis, J.B., D. Smart, and M.J. Gunthorpe. 2002. The vanilloid receptors and vanilloid receptor-like genes: a hot topic getting hotter. Cell Transmissions. 18:3–9.
-
- Diamant, H., M. Funakoshi, L. Strom, and Y. Zotterman. 1963. Electrophysiological studies on human taste nerves. Olfaction and Taste I. Y. Zotterman, editor. Pergamon Press, Oxford. 193–203.
-
- DeSimone, J.A., V. Lyall, G.L. Heck, T.H.T. Phan, R.I. Alam, G.M. Feldman, and R.M. Buch. 2001. A novel pharmacological probe links the amiloride-insensitive NaCl, KCl, and NH4Cl chorda tympani taste responses. J. Neurophysiol. 86:2638–2641. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

