Tissue distribution and excretion of 125I-lidamycin in mice and rats
- PMID: 15929183
- PMCID: PMC4316064
- DOI: 10.3748/wjg.v11.i21.3281
Tissue distribution and excretion of 125I-lidamycin in mice and rats
Abstract
Aim: To investigate the tissue distribution, urinary and fecal excretions of (125)I-lidamycin ((125)I-C-1027) in mice and its biliary excretion in rats.
Methods: The total radioactivity assay (RA method) and the radioactivity assay after precipitation with 200 mL/L trichloroacetic acid (TCA-RA method) were used to determine the tissue distribution, and the urinary and fecal excretions of (125)I-C-1027 in mice and its biliary excretion in rats.
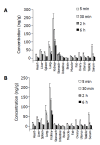
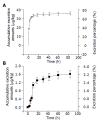
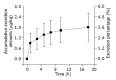
Results: Tissue concentrations reached the peak at the fifth minute after administration of (125)I-C-1027 to mice. The highest concentration was in kidney, and the lowest in brain at all test-time points. The organs of the concentrations of (125)I-C-1027 from high to low were kidney, lung, liver, stomach, spleen, uterus, ovary, intestine, muscle, heart, testis, fat, and brain in mice. The accumulative excretion amounts of 0-24 h, and 0-96 h after administration of (125)I-C-1027 were 68.36 and 71.64% in urine, and 2.60 and 3.21% in feces of mice, respectively, and the accumulative excretion amount of 0-24 h was 3.57% in bile in rats.
Conclusion: Our results reflect the characteristics of the tissue distribution, urinary and fecal excretions of (125)I-C-1027 in mice and the biliary excretion of (125)I-C-1027 and its metabolites in rats, and indicate that (125)I-C-1027 and its metabolites are mainly distributed in kidney, and excreted in urine.
Figures
Similar articles
-
Pharmacokinetics of C-1027 in mice as determined by TCA-RA method.World J Gastroenterol. 2005 Feb 7;11(5):717-20. doi: 10.3748/wjg.v11.i5.717. World J Gastroenterol. 2005. PMID: 15655829 Free PMC article.
-
Comparison of the pharmacokinetics of lidamycin in mice determined by two methods.Yao Xue Xue Bao. 2004 Sep;39(9):695-9. Yao Xue Xue Bao. 2004. PMID: 15606016
-
[Studies on absorption, distribution and excretion of 14C labeled (+-)-7-(3-amino-1-pyrrolidinyl)-6-fluoro-1-(2,4-difluorophenyl)-1,4- dihydro-4-oxo-1,8-naphthyridine-3-carboxylic acid p-toluene-sulfonate hydrate (14C-T-3262) in rats and mice].Jpn J Antibiot. 1989 Apr;42(4):854-67. Jpn J Antibiot. 1989. PMID: 2769938 Japanese.
-
Study on the metabolic fate of catena-(S)-[mu-[N alpha-(3- aminopropionyl)histidinato(2-)-N1,N2,O:N tau]-zinc]. 1st communication: absorption, distribution, metabolism and excretion after single administration to rats.Arzneimittelforschung. 1991 Sep;41(9):965-75. Arzneimittelforschung. 1991. PMID: 1796927
-
Metabolism of dibucaine. II. Disposition and metabolism of dibucaine in rats.J Pharmacobiodyn. 1989 Sep;12(9):523-9. doi: 10.1248/bpb1978.12.523. J Pharmacobiodyn. 1989. PMID: 2614642
Cited by
-
Anticancer activity of natural and synthetic acetylenic lipids.Lipids. 2006 Oct;41(10):883-924. doi: 10.1007/s11745-006-5044-3. Lipids. 2006. PMID: 17180879 Review.
-
Direct in vivo injection of 131I-GMS and its distribution and excretion in rabbit.World J Gastroenterol. 2010 May 7;16(17):2120-8. doi: 10.3748/wjg.v16.i17.2120. World J Gastroenterol. 2010. PMID: 20440852 Free PMC article.
-
Interventional therapy for human breast cancer in nude mice with 131I gelatin microspheres (¹³¹I-GMSs) following intratumoral injection.Radiat Oncol. 2014 Jun 23;9:144. doi: 10.1186/1748-717X-9-144. Radiat Oncol. 2014. PMID: 24958442 Free PMC article.
References
-
- Hu JL, Xue YC, Xie MY, Zhang R, Otani T, Minami Y, Yamada Y, Marunaka T. A new macromolecular antitumor antibiotic, C-1027. I. Discovery, taxonomy of producing organism, fermentation and biological activity. J Antibiot (Tokyo) 1988;41:1575–1579. - PubMed
-
- Otani T, Minami Y, Marunaka T, Zhang R, Xie MY. A new macromolecular antitumor antibiotic, C-1027. II. Isolation and physico-chemical properties. J Antibiot (Tokyo) 1988;41:1580–1585. - PubMed
-
- Zhen YS, Ming XY, Yu B, Otani T, Saito H, Yamada Y. A new macromolecular antitumor antibiotic, C-1027. III. Antitumor activity. J Antibiot (Tokyo) 1989;42:1294–1298. - PubMed
-
- Matsumoto T, Okuno Y, Sugiura Y. Specific interaction between a novel enediyne chromophore and apoprotein in macromolecular antitumor antibiotic C-1027. Biochem Biophys Res Commun. 1993;195:659–666. - PubMed
-
- Otani T. Conformation studies on and assessment by spectral analysis of the protein-chromophore interaction of the macromolecular antitumor antibiotic C-1027. J Antibiot (Tokyo) 1993;46:791–802. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

