Lead exposure inhibits fracture healing and is associated with increased chondrogenesis, delay in cartilage mineralization, and a decrease in osteoprogenitor frequency
- PMID: 15929899
- PMCID: PMC1257601
- DOI: 10.1289/ehp.7596
Lead exposure inhibits fracture healing and is associated with increased chondrogenesis, delay in cartilage mineralization, and a decrease in osteoprogenitor frequency
Abstract
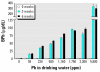
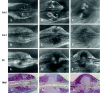
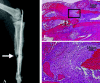
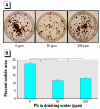
Lead exposure continues to be a significant public health problem. In addition to acute toxicity, Pb has an extremely long half-life in bone. Individuals with past exposure develop increased blood Pb levels during periods of high bone turnover or resorption. Pb is known to affect osteoblasts, osteoclasts, and chondrocytes and has been associated with osteoporosis. However, its effects on skeletal repair have not been studied. We exposed C57/B6 mice to various concentrations of Pb acetate in their drinking water to achieve environmentally relevant blood Pb levels, measured by atomic absorption. After exposure for 6 weeks, each mouse underwent closed tibia fracture. Radiographs were followed and histologic analysis was performed at 7, 14, and 21 days. In mice exposed to low Pb concentrations, fracture healing was characterized by a delay in bridging cartilage formation, decreased collagen type II and type X expression at 7 days, a 5-fold increase in cartilage formation at day 14 associated with delayed maturation and calcification, and a persistence of cartilage at day 21. Fibrous nonunions at 21 days were prevalent in mice receiving very high Pb exposures. Pb significantly inhibited ex vivo bone nodule formation but had no effect on osteoclasts isolated from Pb-exposed animals. No significant effects on osteoclast number or activity were observed. We conclude that Pb delays fracture healing at environmentally relevant doses and induces fibrous nonunions at higher doses by inhibiting the progression of endochondral ossification.
Figures




References
-
- Barnes GL, Kostenuik PJ, Gerstenfeld LC, Einhorn TA. Growth factor regulation of fracture repair. J Bone Miner Res. 1999;14:1805–1815. - PubMed
-
- Berlin K, Gerhardsson S, Borjesson J, Lindh E, Sundstrom N, Schutz A, et al. Lead intoxication caused skeletal disease. Scand J Work Environ Health. 1995;21:296–300. - PubMed
-
- Bonnarens F, Einhorn TA. Production of a standard closed fracture in laboratory animal bone. J Orthop Res. 1984;2:97–101. - PubMed
-
- Bonucci E, Barckhaus RH, Silvestrini G, Ballanti P, Di Lorenzo G. Osteoclast changes induced by lead poisoning (saturnism) Appl Pathol. 1983;1:241–250. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

