Polyarginine as a multifunctional fusion tag
- PMID: 15930002
- PMCID: PMC2253384
- DOI: 10.1110/ps.051393805
Polyarginine as a multifunctional fusion tag
Abstract
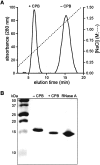
Fusion to cationic peptides, such as nonaarginine (R(9)), provides a means to deliver molecular cargo into mammalian cells. Here, we provide a thorough analysis of the effect of an R(9) tag on the attributes of a model protein: bovine pancreatic ribonuclease (RNase A). The R(9) tag diminishes the conformational stability of RNase A (DeltaT(m)=-8 degrees C in phosphate-buffered saline). This effect is nearly mitigated by the addition of salt. The tag does not compromise the enzymatic activity of RNase A. An R(9) tag facilitates the purification of RNase A by cation-exchange chromatography and enables the adsorption of RNase A on glass slides and silica resin with the retention of enzymatic activity. The tag can be removed precisely and completely by treatment with carboxypeptidase B. Finally, the R(9) tag increases both the cellular uptake of RNase A and the cytotoxicity of G88R RNase A, a variant that evades the cytosolic ribonuclease inhibitor protein. Thus, we conclude that polyarginine is a versatile protein fusion tag.
Figures




References
-
- Antignani, A., Naddeo, M., Cubellis, M.V., Russo, A., and D’Alessio, G. 2001. Antitumor action of seminal ribonuclease, its dimeric structure, and its resistance to the cytosolic ribonuclease inhibitor. Biochemistry 40 3492–3496. - PubMed
-
- Beerens, A.M., Al Hadithy, A.F., Rots, M.G., and Haisma, H.J. 2003. Protein transduction domains and their utility in gene therapy. Curr. Gene Ther. 3 486–494. - PubMed
-
- Bjellqvist, B., Hughes, G.J., Pasquali, C., Paquet, N., Ravier, F., Sanchez, J.C., Frutiger, S., and Hochstrasser, D. 1993. The focusing positions of polypeptides in immobilized pH gradients can be predicted from their amino acid sequences. Electrophoresis 14 1023–1031. - PubMed
-
- Bjellqvist, B., Basse, B., Olsen, E., and Celis, J.E. 1994. Reference points for comparisons of two-dimensional maps of proteins from different human cell types defined in a pH scale where isoelectric points correlate with polypeptide compositions. Electrophoresis 15 529–539. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

