Crystal structure of the protease-resistant core domain of Yersinia pestis virulence factor YopR
- PMID: 15930010
- PMCID: PMC2253397
- DOI: 10.1110/ps.051446405
Crystal structure of the protease-resistant core domain of Yersinia pestis virulence factor YopR
Abstract
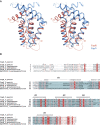
Yersinia pestis, the causative agent of the plague, employs a type III secretion system (T3SS) to secrete and translocate virulence factors into to the cytoplasm of mammalian host cells. One of the secreted virulence factors is YopR. Little is known about the function of YopR other than that it is secreted into the extracellular milieu during the early stages of infection and that it contributes to virulence. Hoping to gain some insight into the function of YopR, we determined the crystal structure of its protease-resistant core domain, which consists of residues 38-149 out of 165 amino acids. The core domain is composed of five alpha-helices that display unexpected structural similarity with one domain of YopN, a central regulator of type III secretion in Y. pestis. This finding raises the possibility that YopR may play a role in the regulation of type III secretion.
Figures
References
-
- Allaoui, A., Schulte, R., and Cornelis, G.R. 1995. Mutational analysis of the Yersinia enterocolitica virC operon: Characterization of yscE, F, G, I, J, K required for Yop secretion and yscH encoding YopR. Mol. Microbiol. 18 343–355. - PubMed
-
- Brunger, A.T. 1992. Free R value: A novel statistical quantity for assessing the accuracy of crystal structures. Nature 355 472–475. - PubMed
-
- Chayen, N.E. 1997. The role of oil in macromolecular crystallization. Structure 5 1269–1274. - PubMed
-
- Collaborative Computational Project 4. 1994. The CCP4 Suite: Programs for Protein Crystallography. Acta Crystallogr. D Biol. Crystallogr. 50 760–763. - PubMed
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources

