Inhibition of translation initiation by volatile anesthetics involves nutrient-sensitive GCN-independent and -dependent processes in yeast
- PMID: 15930127
- PMCID: PMC1182311
- DOI: 10.1091/mbc.e05-02-0127
Inhibition of translation initiation by volatile anesthetics involves nutrient-sensitive GCN-independent and -dependent processes in yeast
Abstract
Volatile anesthetics including isoflurane affect all cells examined, but their mechanisms of action remain unknown. To investigate the cellular basis of anesthetic action, we are studying Saccharomyces cerevisiae mutants altered in their response to anesthetics. The zzz3-1 mutation renders yeast isoflurane resistant and is an allele of GCN3. Gcn3p functions in the evolutionarily conserved general amino acid control (GCN) pathway that regulates protein synthesis and gene expression in response to nutrient availability through phosphorylation of the alpha subunit of eukaryotic initiation factor 2 (eIF2alpha). Hyperphosphorylation of eIF2alpha inhibits translation initiation during amino acid starvation. Isoflurane rapidly (in <15 min) inhibits yeast cell division and amino acid uptake. Unexpectedly, phosphorylation of eIF2alpha decreased dramatically upon initial exposure although hyperphosphorylation occurred later. Translation initiation was inhibited by isoflurane even when eIF2alpha phosphorylation decreased and this inhibition was GCN-independent. Maintenance of inhibition required GCN-dependent hyperphosphorylation of eIF2alpha. Thus, two nutrient-sensitive stages displaying unique features promote isoflurane-induced inhibition of translation initiation. The rapid phase is GCN-independent and apparently has not been recognized previously. The maintenance phase is GCN-dependent and requires inhibition of general translation imparted by enhanced eIF2alpha phosphorylation. Surprisingly, as shown here, the transcription activator Gcn4p does not affect anesthetic response.
Figures
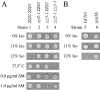




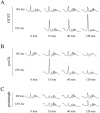
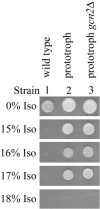
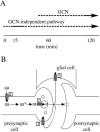
Similar articles
-
Membrane stress is coupled to a rapid translational control of gene expression in chlorpromazine-treated cells.Curr Genet. 2007 Sep;52(3-4):171-85. doi: 10.1007/s00294-007-0151-0. Epub 2007 Aug 21. Curr Genet. 2007. PMID: 17710403
-
PKR and GCN2 kinases and guanine nucleotide exchange factor eukaryotic translation initiation factor 2B (eIF2B) recognize overlapping surfaces on eIF2alpha.Mol Cell Biol. 2005 Apr;25(8):3063-75. doi: 10.1128/MCB.25.8.3063-3075.2005. Mol Cell Biol. 2005. PMID: 15798194 Free PMC article.
-
Heterologous expression of membrane and soluble proteins derepresses GCN4 mRNA translation in the yeast Saccharomyces cerevisiae.Eukaryot Cell. 2006 Feb;5(2):248-61. doi: 10.1128/EC.5.2.248-261.2006. Eukaryot Cell. 2006. PMID: 16467466 Free PMC article.
-
Regulation of translation initiation by amino acids in eukaryotic cells.Prog Mol Subcell Biol. 2001;26:155-84. doi: 10.1007/978-3-642-56688-2_6. Prog Mol Subcell Biol. 2001. PMID: 11575165 Review.
-
Origin of translational control by eIF2α phosphorylation: insights from genome-wide translational profiling studies in fission yeast.Curr Genet. 2021 Jun;67(3):359-368. doi: 10.1007/s00294-020-01149-w. Epub 2021 Jan 9. Curr Genet. 2021. PMID: 33420908 Free PMC article. Review.
Cited by
-
Fusel alcohols regulate translation initiation by inhibiting eIF2B to reduce ternary complex in a mechanism that may involve altering the integrity and dynamics of the eIF2B body.Mol Biol Cell. 2010 Jul 1;21(13):2202-16. doi: 10.1091/mbc.e09-11-0962. Epub 2010 May 5. Mol Biol Cell. 2010. PMID: 20444979 Free PMC article.
-
Anesthetic pretreatment confers thermotolerance on Saccharomyces cerevisiae yeast.Biochem Biophys Res Commun. 2020 Feb 5;522(2):479-484. doi: 10.1016/j.bbrc.2019.11.083. Epub 2019 Nov 25. Biochem Biophys Res Commun. 2020. PMID: 31780265 Free PMC article.
-
Glucose depletion inhibits translation initiation via eIF4A loss and subsequent 48S preinitiation complex accumulation, while the pentose phosphate pathway is coordinately up-regulated.Mol Biol Cell. 2011 Sep;22(18):3379-93. doi: 10.1091/mbc.E11-02-0153. Epub 2011 Jul 27. Mol Biol Cell. 2011. PMID: 21795399 Free PMC article.
-
Role of Saccharomyces cerevisiae TAN1 (tRNA acetyltransferase) in eukaryotic initiation factor 2B (eIF2B)-mediated translation control and stress response.3 Biotech. 2017 Jul;7(3):223. doi: 10.1007/s13205-017-0857-8. Epub 2017 Jul 4. 3 Biotech. 2017. PMID: 28677085 Free PMC article.
-
Local Anesthetics and Antipsychotic Phenothiazines Interact Nonspecifically with Membranes and Inhibit Hexose Transporters in Yeast.Genetics. 2016 Mar;202(3):997-1012. doi: 10.1534/genetics.115.183806. Epub 2016 Jan 12. Genetics. 2016. PMID: 26757771 Free PMC article.
References
-
- Batai, I., Kerenyi, M., and Tekeres, M. (1999). The impact of drugs used in anaesthesia on bacteria. Eur. J. Anaesth. 16, 425-440. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

