Hsp70 protects mitotic cells against heat-induced centrosome damage and division abnormalities
- PMID: 15930131
- PMCID: PMC1182315
- DOI: 10.1091/mbc.e05-01-0038
Hsp70 protects mitotic cells against heat-induced centrosome damage and division abnormalities
Abstract
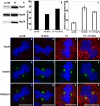
The effect of heat shock on centrosomes has been mainly studied in interphase cells. Centrosomes play a key role in proper segregation of DNA during mitosis. However, the direct effect and consequences of heat shock on mitotic cells and a possible cellular defense system against proteotoxic stress during mitosis have not been described in detail. Here, we show that mild heat shock, applied during mitosis, causes loss of dynamitin/p50 antibody staining from centrosomes and kinetochores. In addition, it induces division errors in most cells and in the remaining cells progression through mitosis is delayed. Expression of heat shock protein (Hsp)70 protects against most heat-induced division abnormalities. On heat shock, Hsp70 is rapidly recruited to mitotic centrosomes and normal progression through mitosis is observed immediately after release of Hsp70 from centrosomes. In addition, Hsp70 expression coincides with restoration of dynamitin/p50 antibody staining at centrosomes but not at kinetochores. Our data show that during mitosis, centrosomes are particularly affected resulting in abnormal mitosis. Hsp70 is sufficient to protect against most division abnormalities, demonstrating the involvement of Hsp70 in a repair mechanism of heat-damaged mitotic centrosomes.
Figures

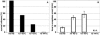




Similar articles
-
HSP70 regulates the function of mitotic centrosomes.Cell Mol Life Sci. 2016 Oct;73(20):3949-60. doi: 10.1007/s00018-016-2236-8. Epub 2016 Apr 30. Cell Mol Life Sci. 2016. PMID: 27137183 Free PMC article.
-
The HspA2 protein localizes in nucleoli and centrosomes of heat shocked cancer cells.J Cell Biochem. 2008 Aug 15;104(6):2193-206. doi: 10.1002/jcb.21778. J Cell Biochem. 2008. PMID: 18452162
-
Centrosome age regulates kinetochore-microtubule stability and biases chromosome mis-segregation.Elife. 2015 Aug 19;4:e07909. doi: 10.7554/eLife.07909. Elife. 2015. PMID: 26287477 Free PMC article.
-
Deregulation of the centrosome cycle and the origin of chromosomal instability in cancer.Adv Exp Med Biol. 2005;570:393-421. doi: 10.1007/1-4020-3764-3_14. Adv Exp Med Biol. 2005. PMID: 18727509 Review.
-
A tale of too many centrosomes.Cell. 2008 Aug 22;134(4):572-5. doi: 10.1016/j.cell.2008.08.007. Cell. 2008. PMID: 18724931 Review.
Cited by
-
Stress-induced localization of HSPA6 (HSP70B') and HSPA1A (HSP70-1) proteins to centrioles in human neuronal cells.Cell Stress Chaperones. 2014 May;19(3):321-7. doi: 10.1007/s12192-013-0459-2. Epub 2013 Sep 6. Cell Stress Chaperones. 2014. PMID: 24061851 Free PMC article.
-
Transcriptional response to stress in the dynamic chromatin environment of cycling and mitotic cells.Proc Natl Acad Sci U S A. 2013 Sep 3;110(36):E3388-97. doi: 10.1073/pnas.1305275110. Epub 2013 Aug 19. Proc Natl Acad Sci U S A. 2013. PMID: 23959860 Free PMC article.
-
Role of Heat Shock Proteins (HSP70 and HSP90) in Viral Infection.Int J Mol Sci. 2021 Aug 29;22(17):9366. doi: 10.3390/ijms22179366. Int J Mol Sci. 2021. PMID: 34502274 Free PMC article. Review.
-
Loss of DIAPH3, a Formin Family Protein, Leads to Cytokinetic Failure Only under High Temperature Conditions in Mouse FM3A Cells.Int J Mol Sci. 2020 Nov 11;21(22):8493. doi: 10.3390/ijms21228493. Int J Mol Sci. 2020. PMID: 33187357 Free PMC article.
-
Polarised asymmetric inheritance of accumulated protein damage in higher eukaryotes.PLoS Biol. 2006 Dec;4(12):e417. doi: 10.1371/journal.pbio.0040417. PLoS Biol. 2006. PMID: 17147470 Free PMC article.
References
-
- Barrau, M. D., Blackburn, G. R., and Dewey, W. C. (1978). Effects of heat on the centrosomes of Chinese hamster ovary (CHO) cells. Cancer Res. 38, 2290-2294. - PubMed
-
- Bharadwaj, R., Qi, W., and Yu, H. (2004). Identification of two novel components of the human NDC80 kinetochore complex. J. Biol. Chem. 279, 13076-13085. - PubMed
-
- Borrelli, M. J., Stafford, D. M., Karczewski, L. A., Rausch, C. M., Lee, Y. J., and Corry, P. M. (1996). Thermotolerance expression in mitotic CHO cells without increased translation of heat shock proteins. J. Cell Physiol. 169, 420-428. - PubMed
-
- Brown, C. R., Doxsey, S. J., Hong-Brown, L. Q., Martin, R. L., and Welch, W. J. (1996a). Molecular chaperones and the centrosome. A role for TCP-1 in microtubule nucleation. J. Biol. Chem. 271, 824-832. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

