Functional role of syndecan-1 cytoplasmic V region in lamellipodial spreading, actin bundling, and cell migration
- PMID: 15930135
- PMCID: PMC1182307
- DOI: 10.1091/mbc.e04-10-0907
Functional role of syndecan-1 cytoplasmic V region in lamellipodial spreading, actin bundling, and cell migration
Erratum in
- Mol Biol Cell. 2005 Oct;16(10):5053
Abstract
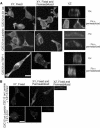
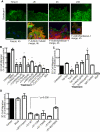
Cell protrusions contribute to cell motility and migration by mediating the outward extension and initial adhesion of cell edges. In many cells, these extensions are supported by actin bundles assembled by the actin cross-linking protein, fascin. Multiple extracellular cues regulate fascin and here we focus on the mechanism by which the transmembrane proteoglycan, syndecan-1, specifically activates lamellipodial cell spreading and fascin-and-actin bundling when clustered either by thrombospondin-1, laminin, or antibody to the syndecan-1 extracellular domain. There is almost no knowledge of the signaling mechanisms of syndecan-1 cytoplasmic domain and we have tested the hypothesis that the unique V region of syndecan-1 cytoplasmic domain has a crucial role in these processes. By four criteria--the activities of N-cadherin/V region chimeras, syndecan-1 deletion mutants, or syndecan-1 point mutants, and specific inhibition by a membrane-permeable TAT-V peptide--we demonstrate that the V region is necessary and sufficient for these cell behaviors and map the molecular basis for its activity to multiple residues located across the V region. These activities correlate with a V-region-dependent incorporation of cell-surface syndecan-1 into a detergent-insoluble form. We also demonstrate functional roles of syndecan-1 V region in laminin-dependent C2C12 cell adhesion and three-dimensional cell migration. These data identify for the first time specific cell behaviors that depend on signaling through the V region of syndecan-1.
Figures

Similar articles
-
A role for syndecan-1 in coupling fascin spike formation by thrombospondin-1.J Cell Biol. 2001 Mar 19;152(6):1169-82. doi: 10.1083/jcb.152.6.1169. J Cell Biol. 2001. PMID: 11257118 Free PMC article.
-
The cytoplasmic domain of syndecan-1 is required for cytoskeleton association but not detergent insolubility. Identification of essential cytoplasmic domain residues.J Biol Chem. 1996 Jun 21;271(25):15253-60. doi: 10.1074/jbc.271.25.15253. J Biol Chem. 1996. PMID: 8662979
-
Syndecan-1 transmembrane and extracellular domains have unique and distinct roles in cell spreading.J Biol Chem. 2003 Nov 21;278(47):46607-15. doi: 10.1074/jbc.M304775200. Epub 2003 Sep 14. J Biol Chem. 2003. PMID: 12975379
-
Syndecan, a developmentally regulated cell surface proteoglycan that binds extracellular matrix and growth factors.Philos Trans R Soc Lond B Biol Sci. 1990 Mar 12;327(1239):171-86. doi: 10.1098/rstb.1990.0052. Philos Trans R Soc Lond B Biol Sci. 1990. PMID: 1969657 Review.
-
Syndecans: multifunctional cell-surface co-receptors.Biochem J. 1997 Oct 1;327 ( Pt 1)(Pt 1):1-16. doi: 10.1042/bj3270001. Biochem J. 1997. PMID: 9355727 Free PMC article. Review.
Cited by
-
The adaptive remodeling of endothelial glycocalyx in response to fluid shear stress.PLoS One. 2014 Jan 20;9(1):e86249. doi: 10.1371/journal.pone.0086249. eCollection 2014. PLoS One. 2014. PMID: 24465988 Free PMC article.
-
A cytoplasmic C-terminal fragment of Syndecan-1 is generated by sequential proteolysis and antagonizes Syndecan-1 dependent lung tumor cell migration.Oncotarget. 2015 Oct 13;6(31):31295-312. doi: 10.18632/oncotarget.5174. Oncotarget. 2015. PMID: 26378057 Free PMC article.
-
Syndecans as Cell Surface Receptors in Cancer Biology. A Focus on their Interaction with PDZ Domain Proteins.Front Pharmacol. 2016 Feb 2;7:10. doi: 10.3389/fphar.2016.00010. eCollection 2016. Front Pharmacol. 2016. PMID: 26869927 Free PMC article. Review.
-
Thrombospondins: Conserved mediators and modulators of metazoan extracellular matrix.Int J Exp Pathol. 2024 Oct;105(5):136-169. doi: 10.1111/iep.12517. Epub 2024 Sep 12. Int J Exp Pathol. 2024. PMID: 39267379 Review.
-
Syndecans shed their reputation as inert molecules.Sci Signal. 2009 Mar 31;2(64):pe18. doi: 10.1126/scisignal.264pe18. Sci Signal. 2009. PMID: 19336838 Free PMC article. Review.
References
-
- Adams, J. C. (1995). Formation of stable microspikes containing actin and the 55kDa actin-bundling protein, fascin, is a cosequence of cell adhesion to thrombospondin-1: implications for the anti-adhesive activities of thrombospondin-1. J. Cell Sci. 108, 1977–1990. - PubMed
-
- Adams, J. C. (2002). Regulation of protrusive and contractile cell-matrix contacts. J. Cell Sci. 115, 257–265. - PubMed
-
- Adams, J. C. (2004). Roles of fascin in cell adhesion and motility. Curr. Opin. Cell Biol. 16, 590–596. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

