Insights into Lafora disease: malin is an E3 ubiquitin ligase that ubiquitinates and promotes the degradation of laforin
- PMID: 15930137
- PMCID: PMC1150849
- DOI: 10.1073/pnas.0503285102
Insights into Lafora disease: malin is an E3 ubiquitin ligase that ubiquitinates and promotes the degradation of laforin
Abstract
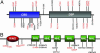
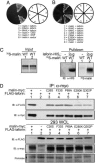
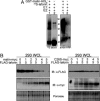
Lafora disease (LD) is a fatal form of progressive myoclonus epilepsy caused by recessive mutations in either a gene encoding a dual-specificity phosphatase, known as laforin, or a recently identified gene encoding the protein known as malin. Here, we demonstrate that malin is a single subunit E3 ubiquitin (Ub) ligase and that its RING domain is necessary and sufficient to mediate ubiquitination. Additionally, malin interacts with and polyubiquitinates laforin, leading to its degradation. Missense mutations in malin that are present in LD patients abolish its ability to polyubiquitinate and signal the degradation of laforin. Our results demonstrate that laforin is a physiologic substrate of malin, and we propose possible models to explain how recessive mutations in either malin or laforin result in LD. Furthermore, these data distinguish malin as an E3 Ub ligase whose activity is necessary to prevent a neurodegenerative disease that involves formation of nonproteinacious inclusion bodies.
Figures


References
-
- Lehesjoki, A. E. (2002) Adv. Neurol. 89, 193-197. - PubMed
-
- Van Heycop Ten Ham, M. W. (1975) in Handbook of Clinical Neurology, eds. Vinken, P. J. & Bruyn, G. W. (North–Holland, Amsterdam), Vol. 15, pp. 382-422.
-
- Berkovic, S. F., Andermann, F., Carpenter, S. & Wolfe, L. S. (1986) N. Engl. J. Med. 315, 296-305. - PubMed
-
- Minassian, B. A. (2002) Adv. Neurol. 89, 199-210. - PubMed
-
- Lafora, G. A. B. G. (1911) Z. Ges. Neurol. Psychiatr. 6, 1-14.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

