Dominant-negative PKC-epsilon impairs apical actin remodeling in parallel with inhibition of carbachol-stimulated secretion in rabbit lacrimal acini
- PMID: 15930141
- PMCID: PMC1414898
- DOI: 10.1152/ajpcell.00546.2004
Dominant-negative PKC-epsilon impairs apical actin remodeling in parallel with inhibition of carbachol-stimulated secretion in rabbit lacrimal acini
Abstract
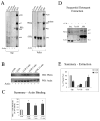
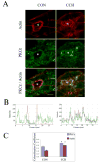
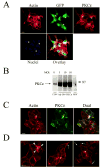
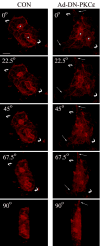
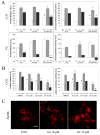
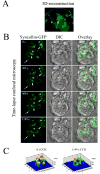
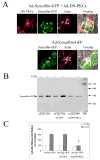
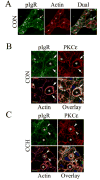
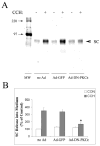
We investigated the involvement of PKC-epsilon in apical actin remodeling in carbachol-stimulated exocytosis in reconstituted rabbit lacrimal acinar cells. Lacrimal acinar PKC-epsilon cosedimented with actin filaments in an actin filament binding assay. Stimulation of acini with carbachol (100 microM, 2-15 min) significantly (P < or = 0.05) increased PKC-epsilon recovery with actin filaments in two distinct biochemical assays, and confocal fluorescence microscopy showed a significant increase in PKC-epsilon association with apical actin in stimulated acini as evidenced by quantitative colocalization analysis. Overexpression of dominant-negative (DN) PKC-epsilon in lacrimal acini with replication-defective adenovirus (Ad) resulted in profound alterations in apical and basolateral actin filaments while significantly inhibiting carbachol-stimulated secretion of bulk protein and beta-hexosaminidase. The chemical inhibitor GF-109203X (10 microM, 3 h), which inhibits PKC-alpha, -beta, -delta, and -epsilon, also elicited more potent inhibition of carbachol-stimulated secretion relative to Gö-6976 (10 microM, 3 h), which inhibits only PKC-alpha and -beta. Transduction of lacrimal acini with Ad encoding syncollin-green fluorescent protein (GFP) resulted in labeling of secretory vesicles that were discharged in response to carbachol stimulation, whereas cotransduction of acini with Ad-DN-PKC-epsilon significantly inhibited carbachol-stimulated release of syncollin-GFP. Carbachol also increased the recovery of secretory component in culture medium, whereas Ad-DN-PKC-epsilon transduction suppressed its carbachol-stimulated release. We propose that DN-PKC-epsilon alters lacrimal acinar apical actin remodeling, leading to inhibition of stimulated exocytosis and transcytosis.
Figures
References
-
- Akita Y, Ohno S, Yajima Y, Konno Y, Saido TC, Mizuno K. Overproduction of a Ca2+-independent protein kinase C isozyme, nPKC epsilon, increases the secretion of prolactin from thyrotropin-releasing hormone-stimulated rat pituitary GH4C1 cells. J Biol Chem. 1994;269:4653–3660. - PubMed
-
- Akita Y. Protein kinase C-ɛ (PKC-ɛ): its unique structure and function. J Biochem. 2002;132:847–852. - PubMed
-
- Aksoy E, Goldman M, Willems F. Protein kinase C epsilon: a new target to control inflammation and immune-mediated disorders. Int J Biochem Cell Biol. 2003;36:183–188. - PubMed
-
- Bastani B, Yang L, Baldassare JJ, Pollo DA, Gardner JD. Cellular distribution of isoforms of protein kinase C (PKC) in pancreatic acini. Biochim Biophys Acta. 1995;1269:307–15. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- EY-13949/EY/NEI NIH HHS/United States
- R01 DK056040/DK/NIDDK NIH HHS/United States
- NS-38246/NS/NINDS NIH HHS/United States
- R03 EY013949/EY/NEI NIH HHS/United States
- R01 GM059297/GM/NIGMS NIH HHS/United States
- EY-11386/EY/NEI NIH HHS/United States
- R01 NS038246/NS/NINDS NIH HHS/United States
- R01 EY011386/EY/NEI NIH HHS/United States
- EY-05081/EY/NEI NIH HHS/United States
- GM-59297/GM/NIGMS NIH HHS/United States
- DK-56040/DK/NIDDK NIH HHS/United States
- R01 EY006177/EY/NEI NIH HHS/United States
- EY-06177/EY/NEI NIH HHS/United States
LinkOut - more resources
Full Text Sources

