BRCA2 suppresses cell proliferation via stabilizing MAGE-D1
- PMID: 15930293
- PMCID: PMC3295243
- DOI: 10.1158/0008-5472.CAN-05-0018
BRCA2 suppresses cell proliferation via stabilizing MAGE-D1
Abstract
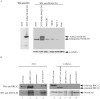
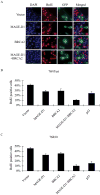
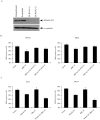
Germ line mutations in BRCA2 gene predispose women to early-onset familial breast and ovarian cancer. BRCA2 is a protein of multiple functions. In addition to its role in DNA double-strand break repair, BRCA2 also plays a role in stabilization of stalled DNA replication forks, cytokinesis, transcription regulation, mammalian gametogenesis, centrosome duplication, and suppression of cell proliferation. However, how BRCA2 mutations predispose women specifically to breast and ovarian cancer remains undefined. Here we found that BRCA2 binds and stabilizes MAGE-D1, a member of the MAGE gene family of proteins. Expression of BRCA2 and MAGE-D1 synergistically suppresses cell proliferation independently of the p53 pathway. Using two MAGE-D1 RNA interferences and two cell lines expressing low or undetectable levels of MAGE-D1, we further showed that the expression of MAGE-D1 is required for BRCA2-mediated suppression of cell proliferation, indicating that MAGE-D1 is a downstream target of BRCA2 and that BRCA2 suppresses cell proliferation via stabilizing MAGE-D1. Importantly, MAGE-D1 protein expression was reduced in 6 of 16 breast carcinoma cell lines tested as compared with untransformed immortal mammary epithelial cell lines, suggesting that suppression of MAGE-D1 expression may be involved in the tumorigenesis of a subset of sporadic breast cancers.
Figures


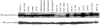
References
-
- Tavtigian SV, Simard J, Rommens J, et al. The complete BRCA2 gene and mutations in chromosome 13q-linked kindreds. Nat Genet. 1996;12:333–7. - PubMed
-
- Wooster R, Neuhausen SL, Mangion J, et al. Localization of a breast cancer susceptibility gene, BRCA2, to chromosome 13q12-13. Science (Wash DC) 1994;265:2088–90. - PubMed
-
- Wooster R, Bignell G, Lancaster J, et al. Identification of the breast cancer susceptibility gene BRCA2. Nature (Lond) 1995;378:789–92. - PubMed
-
- Gayther SA, Pharoah PD, Ponder BA. The genetics of inherited breast cancer. J Mammary Gland Biol Neoplasia. 1998;3:365–76. - PubMed
-
- Sharan SK, Morimatsu M, Albrecht U, et al. Embryonic lethality and radiation hypersensitivity mediated by Rad51 in mice lacking Brca2. Nature (Lond) 1997;386:804–10. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

