Light stimulates MSK1 activation in the suprachiasmatic nucleus via a PACAP-ERK/MAP kinase-dependent mechanism
- PMID: 15930378
- PMCID: PMC6724997
- DOI: 10.1523/JNEUROSCI.4361-04.2005
Light stimulates MSK1 activation in the suprachiasmatic nucleus via a PACAP-ERK/MAP kinase-dependent mechanism
Erratum in
- J Neurosci. 2005 Jun 29;25(26):6261-2
Abstract
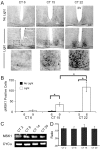
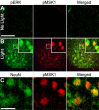
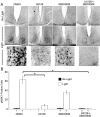
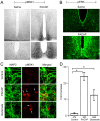
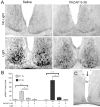
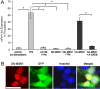
Signaling via the p42/44 mitogen-activated protein kinase (MAPK) pathway has been shown to be a key intracellular signaling event that couples light to entrainment of the mammalian circadian clock located in the suprachiasmatic nucleus (SCN). Because many of the physiological effects of the MAPK pathway are mediated by extracellular signal-regulated kinase (ERK)-regulated kinases, it was of interest to identify kinase targets of ERK in the SCN. In this study, we examined whether mitogen- and stress-activated protein kinase 1 (MSK1) is a downstream target of ERK in the SCN and whether it couples to clock gene expression. Here we show that photic stimulation during the subjective night stimulates MSK1 phosphorylation at serine 360, an event required for robust kinase activation. Activated ERK and MSK1 were colocalized in SCN cell nuclei after photic stimulation. The in vivo administration of the MAP kinase kinase 1/2 inhibitor U0126 [1,4-diamino-2,3-dicyano-1,4-bis(o-aminophenylmercapto) butadiene] attenuated MSK1 phosphorylation. MSK1 phosphorylation was more responsive to late-night than early-night photic stimulation, indicating that MSK1 may differentially contribute to light-induced phase advancing and phase delaying of the clock. The potential connection between pituitary adenylate cyclase-activating polypeptide (PACAP) (a regulator of clock entrainment) and MSK1 phosphorylation was examined. PACAP infusion stimulated MSK1 phosphorylation, whereas PACAP receptor antagonist infusion attenuated light-induced MSK1 phosphorylation in the SCN. In reporter gene assays, MSK1 was shown to couple to mPeriod1 via a cAMP response element-binding protein-dependent mechanism. Together, these data identify MSK1 as both a downstream target of the MAPK cascade within the SCN and a regulator of clock gene expression.
Figures
Similar articles
-
Mitogen- and stress-activated protein kinase 1 modulates photic entrainment of the suprachiasmatic circadian clock.Eur J Neurosci. 2013 Jan;37(1):130-40. doi: 10.1111/ejn.12028. Epub 2012 Nov 6. Eur J Neurosci. 2013. PMID: 23127194 Free PMC article.
-
Light- and clock-dependent regulation of ribosomal S6 kinase activity in the suprachiasmatic nucleus.Eur J Neurosci. 2004 Feb;19(4):907-15. doi: 10.1111/j.0953-816x.2004.03155.x. Eur J Neurosci. 2004. PMID: 15009138
-
Involvement of calcium-calmodulin protein kinase but not mitogen-activated protein kinase in light-induced phase delays and Per gene expression in the suprachiasmatic nucleus of the hamster.J Neurochem. 2001 Apr;77(2):618-27. doi: 10.1046/j.1471-4159.2001.00270.x. J Neurochem. 2001. PMID: 11299324
-
An essential role for peptidergic signalling in the control of circadian rhythms in the suprachiasmatic nuclei.J Neuroendocrinol. 2003 Apr;15(4):335-8. doi: 10.1046/j.1365-2826.2003.01005.x. J Neuroendocrinol. 2003. PMID: 12622830 Review.
-
Roles of PACAP-containing retinal ganglion cells in circadian timing.Int Rev Cytol. 2006;251:1-39. doi: 10.1016/S0074-7696(06)51001-0. Int Rev Cytol. 2006. PMID: 16939776 Review.
Cited by
-
Suprachiasmatic function in a circadian period mutant: Duper alters light-induced activation of vasoactive intestinal peptide cells and PERIOD1 immunostaining.Eur J Neurosci. 2018 Dec;48(11):3319-3334. doi: 10.1111/ejn.14214. Eur J Neurosci. 2018. PMID: 30346078 Free PMC article.
-
microRNA modulation of circadian-clock period and entrainment.Neuron. 2007 Jun 7;54(5):813-29. doi: 10.1016/j.neuron.2007.05.017. Neuron. 2007. PMID: 17553428 Free PMC article.
-
Delayed Effect of the Light Pulse on Phosphorylated ERK1/2 and GSK3β Kinases in the Ventrolateral Suprachiasmatic Nucleus of Rat.J Mol Neurosci. 2015 Jun;56(2):371-6. doi: 10.1007/s12031-015-0563-0. Epub 2015 Apr 17. J Mol Neurosci. 2015. PMID: 25894767
-
Linking neural activity and molecular oscillations in the SCN.Nat Rev Neurosci. 2011 Sep 2;12(10):553-69. doi: 10.1038/nrn3086. Nat Rev Neurosci. 2011. PMID: 21886186 Free PMC article. Review.
-
Circadian expression and functional characterization of PEA-15 within the mouse suprachiasmatic nucleus.Eur J Neurosci. 2018 Apr;47(7):845-857. doi: 10.1111/ejn.13850. Epub 2018 Feb 19. Eur J Neurosci. 2018. PMID: 29383758 Free PMC article.
References
-
- Akiyama M, Kouzu Y, Takahashi S, Wakamatsu H, Moriya T, Maetani M, Watanabe S, Tei H, Sakaki Y, Shibata S (1999) Inhibition of light- or glutamate-induced mPer1 expression represses the phase shifts into the mouse circadian locomotor and suprachiasmatic firing rhythms. J Neurosci 19: 1115-1121. - PMC - PubMed
-
- Albrecht U (2002) Functional genomics of sleep and circadian rhythm. Invited review: regulation of mammalian circadian clock genes. J Appl Physiol 92: 1348-1355. - PubMed
-
- Albrecht U, Sun ZS, Eichele G, Lee CC (1997) A differential response of two putative mammalian circadian regulators, mper1 and mper2, to light. Cell 91: 1055-1064. - PubMed
-
- Albrecht U, Zheng B, Larkin D, Sun ZS, Lee CC (2001) MPer1 and mper2 are essential for normal resetting of the circadian clock. J Biol Rhythms 16: 100-104. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous
