The generation of a 17 kDa neurotoxic fragment: an alternative mechanism by which tau mediates beta-amyloid-induced neurodegeneration
- PMID: 15930385
- PMCID: PMC1352316
- DOI: 10.1523/JNEUROSCI.1125-05.2005
The generation of a 17 kDa neurotoxic fragment: an alternative mechanism by which tau mediates beta-amyloid-induced neurodegeneration
Abstract
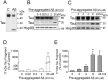
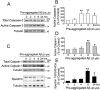
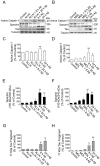
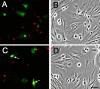
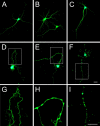
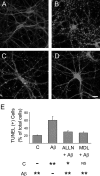
Recently, we have shown that the microtubule-associated protein tau is essential for beta-amyloid (Abeta)-induced neurotoxicity in hippocampal neurons. However, the mechanisms by which tau mediates Abeta-induced neurite degeneration remain poorly understood. In the present study, we analyzed whether tau cleavage played a role in these events. Our results showed that pre-aggregated Abeta induced the generation of a 17 kDa tau fragment in cultured hippocampal neurons. The generation of this fragment was preceded by the activation of calpain-1. Conversely, inhibitors of this protease, but not of caspases, completely prevented tau proteolysis leading to the generation of the 17 kDa fragment and significantly reduced Abeta-induced neuronal death. Furthermore, the expression of this fragment in cultured hippocampal neurons induced the formation of numerous varicosity-bearing tortuous processes, as well as the complete degeneration of some of those neurite processes. These results suggest that Abeta-induced neurotoxicity may be mediated, at least in part, through the calpain-mediated generation of a toxic 17 kDa tau fragment. Collectively, these results provide insight into a novel mechanism by which tau could mediate Abeta-induced neurotoxicity.
Figures
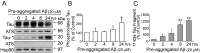
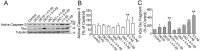

Similar articles
-
Atorvastatin prevents Aβ oligomer-induced neurotoxicity in cultured rat hippocampal neurons by inhibiting Tau cleavage.Acta Pharmacol Sin. 2015 May;36(5):553-64. doi: 10.1038/aps.2014.161. Epub 2015 Apr 20. Acta Pharmacol Sin. 2015. PMID: 25891085 Free PMC article.
-
Caspase-3- and calpain-mediated tau cleavage are differentially prevented by estrogen and testosterone in beta-amyloid-treated hippocampal neurons.Neuroscience. 2007 Jan 5;144(1):119-27. doi: 10.1016/j.neuroscience.2006.09.012. Epub 2006 Oct 19. Neuroscience. 2007. PMID: 17055174 Free PMC article.
-
The novel calpain inhibitor A-705253 potently inhibits oligomeric beta-amyloid-induced dynamin 1 and tau cleavage in hippocampal neurons.Neurochem Int. 2008 Sep;53(3-4):79-88. doi: 10.1016/j.neuint.2008.06.003. Epub 2008 Jun 12. Neurochem Int. 2008. PMID: 18590784 Free PMC article.
-
Caspases in Alzheimer's Disease: Mechanism of Activation, Role, and Potential Treatment.Mol Neurobiol. 2024 Jul;61(7):4834-4853. doi: 10.1007/s12035-023-03847-1. Epub 2023 Dec 23. Mol Neurobiol. 2024. PMID: 38135855 Free PMC article. Review.
-
Acetylcholinesterase interaction with Alzheimer amyloid beta.Subcell Biochem. 2005;38:299-317. doi: 10.1007/0-387-23226-5_15. Subcell Biochem. 2005. PMID: 15709485 Review.
Cited by
-
Supplementation of Convolvulus pluricaulis attenuates scopolamine-induced increased tau and amyloid precursor protein (AβPP) expression in rat brain.Indian J Pharmacol. 2012 Sep-Oct;44(5):593-8. doi: 10.4103/0253-7613.100383. Indian J Pharmacol. 2012. PMID: 23112420 Free PMC article.
-
Cellular factors modulating the mechanism of tau protein aggregation.Cell Mol Life Sci. 2015 May;72(10):1863-79. doi: 10.1007/s00018-015-1839-9. Epub 2015 Feb 11. Cell Mol Life Sci. 2015. PMID: 25666877 Free PMC article. Review.
-
Biochemistry and cell biology of tau protein in neurofibrillary degeneration.Cold Spring Harb Perspect Med. 2012 Jul;2(7):a006247. doi: 10.1101/cshperspect.a006247. Cold Spring Harb Perspect Med. 2012. PMID: 22762014 Free PMC article. Review.
-
The fuzzy coat of pathological human Tau fibrils is a two-layered polyelectrolyte brush.Proc Natl Acad Sci U S A. 2013 Jan 22;110(4):E313-21. doi: 10.1073/pnas.1212100110. Epub 2012 Dec 26. Proc Natl Acad Sci U S A. 2013. PMID: 23269837 Free PMC article.
-
Atorvastatin prevents Aβ oligomer-induced neurotoxicity in cultured rat hippocampal neurons by inhibiting Tau cleavage.Acta Pharmacol Sin. 2015 May;36(5):553-64. doi: 10.1038/aps.2014.161. Epub 2015 Apr 20. Acta Pharmacol Sin. 2015. PMID: 25891085 Free PMC article.
References
-
- Adamec E, Mohan P, Vonsattel JP, Nixon RA (2002a) Calpain activation in neurodegenerative diseases: confocal immunofluorescence study with antibodies specifically recognizing the active form of calpain 2. Acta Neuropathol (Berl) 104: 92-104. - PubMed
-
- Adamec E, Murrell JR, Takao M, Hobbs W, Nixon RA, Ghetti B, Vonsattel JP (2002b) P301L tauopathy: confocal immunofluorescence study of perinuclear aggregation of the mutated protein. J Neurol Sci 200: 85-93. - PubMed
-
- Allen JW, Eldadah BA, Huang X, Knoblach SM, Faden AI (2001) Multiple caspases are involved in beta-amyloid-induced neuronal apoptosis. J Neurosci Res 65: 45-53. - PubMed
-
- Alvarez A, Toro R, Caceres A, Maccioni RB (1999) Inhibition of tau phosphorylating protein kinase cdk5 prevents beta-amyloid-induced neuronal death. FEBS Lett 459: 421-426. - PubMed
-
- Bensadoun A, Weinstein D (1976) Assay of proteins in the presence of interfering materials. Anal Biochem 70: 241-250. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
