ProBDNF induces neuronal apoptosis via activation of a receptor complex of p75NTR and sortilin
- PMID: 15930396
- PMCID: PMC6724992
- DOI: 10.1523/JNEUROSCI.5123-04.2005
ProBDNF induces neuronal apoptosis via activation of a receptor complex of p75NTR and sortilin
Abstract
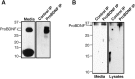
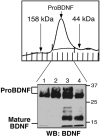
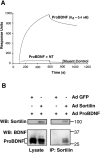
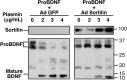
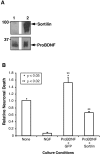
Brain-derived neurotrophic factor (BDNF) is best characterized for critical roles in neuronal survival, differentiation, and synaptic modulation mediated by the TrkB receptor tyrosine kinase. Developmentally regulated death signaling by BDNF has also been demonstrated via activation of p75NTR. Because recent studies suggest that proNGF, the precursor form of NGF, is more active than mature NGF in inducing apoptosis after binding to p75NTR and a coreceptor, sortilin, we asked whether the precursor of BDNF (proBDNF) is also a proapoptotic ligand in the nervous system. proBDNF is secreted by cultured neurons, and recombinant proBDNF binds to sortilin. In sympathetic neurons coexpressing sortilin and p75NTR, we found that proBDNF is an apoptotic ligand that induces death at subnanomolar concentrations. In contrast, mature BDNF, but not proBDNF, is effective in inducing TrkB phosphorylation. proBDNF effects are dependent on cellular coexpression of both p75NTR and sortilin, because neurons deficient in p75NTR are resistant to proBDNF-induced apoptosis, and competitive antagonists of sortilin block sympathetic neuron death. Moreover, addition of preformed complexes of soluble sortilin and proBDNF failed to induce apoptosis of cells coexpressing both sortilin and p75NTR, suggesting that interaction of proBDNF with both receptors on the cell surface is required to initiate cell death. Together with our past findings, these data suggest that the neurotrophin family is capable of modulating diverse biological processes via differential processing of the proneurotrophins.
Figures



References
-
- Ashkenazi A (2002)) Targeting death and decoy receptors of the tumournecrosis factor superfamily. Nat Rev Cancer 2: 420-430. - PubMed
-
- Banfield MJ, Naylor RL, Robertson AG, Allen SJ, Dawbarn D, Brady RL (2001) Specificity in Trk receptor:neurotrophin interactions: the crystal structure of TrkB-d5 in complex with neurotrophin-4/5. Structure 9: 1191-1199. - PubMed
-
- Boyd JG, Gordon T (2002) A dose-dependent facilitation and inhibition of peripheral nerve regeneration by brain-derived neurotrophic factor. Eur J Neurosci 15: 613-626. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous
