Modulation of memory consolidation by the basolateral amygdala or nucleus accumbens shell requires concurrent dopamine receptor activation in both brain regions
- PMID: 15930508
- PMCID: PMC1142458
- DOI: 10.1101/lm.93205
Modulation of memory consolidation by the basolateral amygdala or nucleus accumbens shell requires concurrent dopamine receptor activation in both brain regions
Abstract
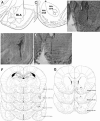
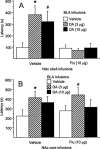
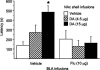
Previous findings indicate that the basolateral amygdala (BLA) and the nucleus accumbens (NAc) interact in influencing memory consolidation. The current study investigated whether this interaction requires concurrent dopamine (DA) receptor activation in both brain regions. Unilateral, right-side cannulae were implanted into the BLA and the ipsilateral NAc shell or core in male Sprague-Dawley rats ( approximately 300 g). One week later, the rats were trained on an inhibitory avoidance (IA) task and, 48 h later, they were tested for retention. Drugs were infused into the BLA and NAc shell or core immediately after training. Post-training intra-BLA infusions of DA enhanced retention, as assessed by latencies to enter the shock compartment on the retention test. Infusions of the general DA receptor antagonist cis-Flupenthixol (Flu) into the NAc shell (but not the core) blocked the memory enhancement induced by the BLA infusions of DA. In the reverse experiment, post-training intra-NAc shell infusions of DA enhanced retention and Flu infusions into the BLA blocked the enhancement. These findings indicate that BLA modulation of memory consolidation requires concurrent DA receptor activation in the NAc shell but not the core. Similarly, NAc shell modulation of memory consolidation requires concurrent DA receptor activation in the BLA. Together with previous findings, these results suggest that the dopaminergic innervation of the BLA and NAc shell is critically involved in the modulation of memory consolidation.
Figures
References
-
- Barrot, M., Marinelli, M., Abrous, D.N., Rouge-Pont, F., Le Moal, M., and Piazza, P.V. 2000. The dopaminergic hyper-responsiveness of the shell of the nucleus accumbens is hormone-dependent. Eur. J. Neurosci. 12: 973-979. - PubMed
-
- Carr, G.D. and White, N.M. 1983. Conditioned place preference from intra-accumbens but not intra-caudate amphetamine injections. Life Sci. 33: 2551-2557. - PubMed
-
- Christie, M.J., Summers, R.J., Stephenson, J.A., Cook, C.J., and Beart, P.M. 1987. Excitatory amino acid projections to the nucleus accumbens septi in the rat: A retrograde transport study utilizing d[3h]aspartate and [3h]gaba. Neuroscience. 22: 425-439. - PubMed
-
- Fallon, J.H. and Loughlin, S.E. 1995. Substantia nigra. In The rat nervous system (ed. G. Paxinos), pp. 215-237. Academic Press, San Diego, CA.
-
- Floresco, S.B., Yang, C.R., Phillips, A.G., and Blaha, C.D. 1998. Basolateral amygdala stimulation evokes glutamate receptor-dependent dopamine efflux in the nucleus accumbens of the anaesthetized rat. Eur. J. Neurosci. 10: 1241-1251. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
