Medical applications of transforming growth factor-beta
- PMID: 15931280
- PMCID: PMC1069016
- DOI: 10.3121/cmr.1.1.13
Medical applications of transforming growth factor-beta
Abstract
Transforming growth factor-beta (TGF-beta) proteins and their antagonists have entered clinical trials. These multi-functional regulators of cell growth and differentiation induce extracellular matrix proteins and suppress the immune system making TGF-betas useful in treatment of wounds with impaired healing, mucositis, fractures, ischemia-reperfusion injuries, and autoimmune disease. In diseases such as keloids, glomerulonephritis and pulmonary fibrosis, excessive expression of TGF-beta has been implicated as being responsible for accumulation of detrimental scar tissue. In these conditions, agents that block TGF-beta have prevented or reversed disease. Similarly, in carcinogenesis, blocking TGF-beta activity may be valuable in stimulating an immune response towards metastasis. As these blocking agents receive approval, we will likely have new therapies for previously recalcitrant diseases.
Figures
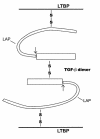
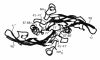
References
-
- Roberts AB, Sporn MB. The transforming growth factor-βs. In: Sporn MB, Roberts AB, editors. Peptide Growth Factors and Their Receptors. Handbook of Experimental Pharmacology. Vol. 1, Chapter 8. Berlin, Heidelberg: Springer; 1995. pp. 419–472.
-
- Massagué J. The transforming growth factor-β family. Annu Rev Cell Biol. 1990;6:597–641. - PubMed
-
- Ten Dijke P, Goumans MJ, Itoh F, Itoh S. Regulation of cell proliferation by Smad proteins. J Cell Physiol. 2002;191:1–16. - PubMed
-
- Verrecchia F, Mauviel A. Transforming growth factor-β signaling through the Smad pathway: Role in extracellular matrix gene expression and regulation. J Invest Dermatol. 2002;118:211–215. - PubMed
-
- Massagué J, Blain SW, Lo RS. TGF-β signaling in growth control, cancer, and heritable disorders. Cell. 2000;103:295–309. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
