Prevention of iron deficiency anemia in adolescent and adult pregnancies
- PMID: 15931282
- PMCID: PMC1069018
- DOI: 10.3121/cmr.1.1.29
Prevention of iron deficiency anemia in adolescent and adult pregnancies
Abstract
Objective: Worldwide attention over iron deficiency anemia (IDA) in pregnancy has shifted recently from providing supplements during pregnancy to attempting to ensure that women, especially adolescents, have adequate iron stores prior to conception. We sought to determine whether adolescent and/or adult women still need supplements during pregnancy to avoid IDA, even if iron stores are adequate, and whether the IDA translates into maternal and/or infant morbidity and mortality.
Design: Randomized, double-blind clinical trial with placebo control.
Setting: Multicenter clinic setting in central Wisconsin.
Participants: Adolescent women 18 years or less in their first pregnancy, and adult women 19 years or older, who were found to be healthy and iron sufficient at their first prenatal visit.
Methods: Participants were randomized to receive iron supplementation (60 mg/day elemental iron) or placebo. Serum ferritin of 12 ng/mL or less with simultaneous hemoglobin of 11 g/dL or less defined IDA. When IDA occurred at the second trimester, a therapeutic supplement of 180 mg of elemental iron per day was initiated.
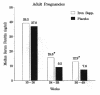
Results: Forty-seven percent of all placebo-supplemented and 16% of all iron-supplemented patients exhibited IDA (p<0.001); 59% of adolescent placebo-supplemented and 20% of adolescent iron-supplemented patients exhibited IDA (p=0.021). Nausea, vomiting, diarrhea, and constipation were not significantly different in the iron supplemented group compared to the placebo group, and no significant differences were seen in maternal or neonatal health, but the number of women studied was limiting for analysis of these adverse events.
Conclusion: IDA is common in healthy, iron-sufficient adolescent pregnant women during the second trimester, and body stores of iron decline in both adolescent and adult pregnancies. The incidence of IDA during adolescent and adult pregnancies is substantially reduced with 60 mg of elemental iron per day. However, there remains no clear evidence that maternal or neonatal health will benefit from correcting these deficits.
Figures

References
-
- Abel R, Rajaratnam J, Kalaimani A, Kirubakaran S. Can iron status be improved in each of the three trimesters? A community-based study. Eur J Clin Nutr. 2000;54:490–493. - PubMed
-
- Allen LH, Rosado JL, Casterline JE, Lopez P, Munoz E, Garcia OP, Martinez H. Lack of hemoglobin response to iron supplementation in anemic Mexican preschoolers with multiple micronutrient deficiencies. Am J Clin Nutr. 2000;71:1485–1494. - PubMed
-
- Baynes RD, Cook JD. Current issues in iron deficiency. Curr Opin Hematol. 1996;3:145–149. - PubMed
-
- Beard JL. Weekly iron intervention: the case for intermittent iron supplementation. Am J Clin Nutr. 1998;68:209–212. - PubMed
-
- Brabin BJ, Hakimi M, Pelletier D. An analysis of anemia and pregnancy-related maternal mortality. J Nutr. 2001;131:604S–614S. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
