Characterization of chito-oligosaccharides prepared by chitosanolysis with the aid of papain and Pronase, and their bactericidal action against Bacillus cereus and Escherichia coli
- PMID: 15932346
- PMCID: PMC1276913
- DOI: 10.1042/BJ20050093
Characterization of chito-oligosaccharides prepared by chitosanolysis with the aid of papain and Pronase, and their bactericidal action against Bacillus cereus and Escherichia coli
Abstract
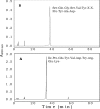
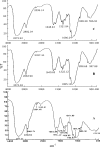
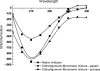
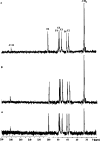
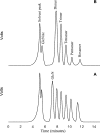
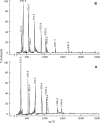
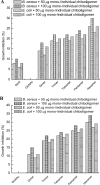
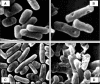
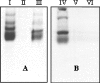
Papain (from papaya latex; EC 3.4.22.2) and Pronase (from Streptomyces griseus; EC 3.4.24.31) caused optimum depolymerization of chitosan at pH 3.5 and 37 degrees C, resulting in LMMC (low molecular mass chitosan) and chito-oligomeric-monomeric mixture. The yield of the latter was 14-16% and 14-19% respectively for papain- and Pronase-catalysed reactions, depending on the reaction time (1-5 h). HPLC revealed the presence of monomer(s) and oligomers of DP (degree of polymerization) 2-6, which was also confirmed by matrix-assisted laser-desorption ionization-time-of-flight MS. Along with the chito-oligomers, the appearance of only GlcNAc (N-acetylglucosamine) in Pronase-catalysed chitosanolysis was indicative of its different action pattern compared with papain. Fourier-transform infrared, liquid-state 13C-NMR spectra and CD analyses of chito-oligomeric-monomeric mixture indicated the release of GlcNAc/GlcNAc-rich oligomers. The monomeric sequence at the non-reducing ends of chito-oligomers was elucidated using N-acetylglucosaminidase. The chito-oligomeric-monomeric mixture showed better growth inhibitory activity towards Bacillus cereus and Escherichia coli compared with native chitosan. Optimum growth inhibition was observed with chito-oligomers of higher DP having low degree of acetylation. The latter caused pore formation and permeabilization of the cell wall of B. cereus, whereas blockage of nutrient flow due to the aggregation of chito-oligomers-monomers was responsible for the growth inhibition and lysis of E. coli, which were evidenced by scanning electron microscopy analysis. The spillage of cytoplasmic enzymes and native PAGE of the cell-free supernatant of B. cereus treated with chito-oligomeric-monomeric mixture further confirmed bactericidal activity of the latter. Use of papain and Pronase, which are inexpensive and easily available, for chitosanolysis, is of commercial importance, as the products released are of considerable biomedical value.
Figures

Similar articles
-
Low molecular weight chitosans--preparation with the aid of pronase, characterization and their bactericidal activity towards Bacillus cereus and Escherichia coli.Biochim Biophys Acta. 2007 Apr;1770(4):495-505. doi: 10.1016/j.bbagen.2006.12.003. Epub 2006 Dec 20. Biochim Biophys Acta. 2007. PMID: 17240531
-
Chitooligosaccharides--preparation with the aid of pectinase isozyme from Aspergillus niger and their antibacterial activity.Carbohydr Res. 2005 May 2;340(6):1239-45. doi: 10.1016/j.carres.2005.02.005. Carbohydr Res. 2005. PMID: 15797142
-
Low molecular weight chitosans: preparation with the aid of papain and characterization.Biochim Biophys Acta. 2004 Jan 22;1670(2):137-46. doi: 10.1016/j.bbagen.2003.11.004. Biochim Biophys Acta. 2004. PMID: 14738997
-
The cell factory approach toward biotechnological production of high-value chitosan oligomers and their derivatives: an update.Crit Rev Biotechnol. 2017 Feb;37(1):11-25. doi: 10.3109/07388551.2015.1104289. Epub 2015 Nov 2. Crit Rev Biotechnol. 2017. PMID: 26526199 Review.
-
Preparation of Defined Chitosan Oligosaccharides Using Chitin Deacetylases.Int J Mol Sci. 2020 Oct 22;21(21):7835. doi: 10.3390/ijms21217835. Int J Mol Sci. 2020. PMID: 33105791 Free PMC article. Review.
Cited by
-
Evaluation of Antibacterial and Antifungal Properties of Low Molecular Weight Chitosan Extracted from Hermetia illucens Relative to Crab Chitosan.Molecules. 2022 Jan 17;27(2):577. doi: 10.3390/molecules27020577. Molecules. 2022. PMID: 35056890 Free PMC article.
-
Antimicrobial activity and chemical composition of essential oil from Helichrysum microphyllum Cambess. subsp. tyrrhenicum Bacch., Brullo & Giusso collected in South-West Sardinia.Saudi J Biol Sci. 2019 Jul;26(5):897-905. doi: 10.1016/j.sjbs.2018.04.009. Epub 2018 Apr 10. Saudi J Biol Sci. 2019. PMID: 31303817 Free PMC article.
-
Antimicrobial Activities of Chitosan Derivatives.Pharmaceutics. 2021 Oct 8;13(10):1639. doi: 10.3390/pharmaceutics13101639. Pharmaceutics. 2021. PMID: 34683932 Free PMC article.
-
Antibacterial characteristics and activity of water-soluble chitosan derivatives prepared by the Maillard reaction.Molecules. 2011 Oct 11;16(10):8504-14. doi: 10.3390/molecules16108504. Molecules. 2011. PMID: 21989311 Free PMC article.
-
Pilot-Scale Production of Chito-Oligosaccharides Using an Innovative Recombinant Chitosanase Preparation Approach.Polymers (Basel). 2021 Jan 18;13(2):290. doi: 10.3390/polym13020290. Polymers (Basel). 2021. PMID: 33477553 Free PMC article.
References
-
- Tharanathan R. N., Kittur F. S. Chitin – the undisputed biomolecule of great potential. Crit. Rev. Food Sci. Nutr. 2003;43:61–87. - PubMed
-
- Shon D. H. Proceedings of the International Workshop on Bioactive Natural Products, Tokyo, Japan. Tokyo, Japan: The Committee on Science and Technology in Developing Countries (COSTED) and the Science Council of Japan; 2001. Chitosan oligosaccharides for functional foods and microbial enrichment of chitosan oligosaccharides in soy-paste. pp. 56–66.
-
- Suzuki K., Mikami T., Okawa Y., Tokoro A., Suzuki S., Suzuki M. Antitumor effects of hexa-N-acetylchitohexaose and chitohexaose. Carbohydr. Res. 1986;151:403–408. - PubMed
-
- Qin C., Du Y., Xiao L., Li Z., Gao X. Enzymic preparation of water-soluble chitosan and their antitumor activity. Int. J. Biol. Macromol. 2003;31:111–117. - PubMed
-
- Ikeda I., Sugano M., Yashida K., Sasaki E., Iwamoto Y., Hatono K. Dietary fibre and lipid absorption: interference of chitosan and its hydrolyzate with cholesterol and fatty acid absorption and metabolic consequences in rats. In: Kritchevsky D., Bonefield C., editors. Dietary Fibre in Health and Disease. St. Paul, MN: Eagan Press; 1995. pp. 95–106.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

