The interaction between staphylococcal superantigen-like proteins and human dendritic cells
- PMID: 15932507
- PMCID: PMC1809393
- DOI: 10.1111/j.1365-2249.2005.02789.x
The interaction between staphylococcal superantigen-like proteins and human dendritic cells
Abstract
Staphylococcus aureus produce a family of exotoxins (staphylococcal superantigen like proteins, SSLs) with structural, but not functional, homology to superantigens. These proteins have previously been shown to interact selectively with antigen presenting cells, including dendritic cells. The functional consequences of this interaction are now explored. SSL7 and 9 had no effect on viability or morphology of dendritic cells. The proteins did not induce dendritic cell maturation, as measured by cell surface phenotype. Exposure to SSL did not alter the ability of dendritic cells to take up FITC-dextran. Finally, exposure to SSLs did not impair the ability of the dendritic cells to stimulate allogeneic or antigen specific T cell responses. However, dendritic cells loaded with SSL7 or 9 were able to stimulate a T cell proliferative response in 3/8 healthy individuals tested. Sera from nine out of 10 individuals tested contained antibodies against both SSL7 and SSL9, and the response to each SSL was specific and not cross-reactive. The results demonstrate that SSLs are immunogenic in humans at both the B and T cell level, but it remains unclear whether this response is to the benefit of the bacterium or the host.
Figures
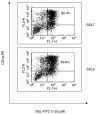
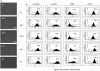
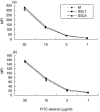
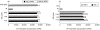
 ) and cultured for 18 h before the addition of autologous T cells (2 × 105) in the presence or absence (M) of purified protein derivative (PPD; 500 U/ml). Data are mean ± SEM of 5 experiments. (b) DCs (104) were treated with different concentrations of SSL7 (▪) or SSL9 (□)(0·42, 1·25 and 4·16 µM) or medium alone (
) and cultured for 18 h before the addition of autologous T cells (2 × 105) in the presence or absence (M) of purified protein derivative (PPD; 500 U/ml). Data are mean ± SEM of 5 experiments. (b) DCs (104) were treated with different concentrations of SSL7 (▪) or SSL9 (□)(0·42, 1·25 and 4·16 µM) or medium alone ( ) and cultured for 18 h before the addition of allogeneic T cells (2 × 105). DC – dendritic cells cultured without allogeneic T cells; TC-allogeneic T cells cultured without dendritic cells. Data are mean ± SEM of 3 experiments. Proliferation was assessed by incorporation of 3H-thymidine.
) and cultured for 18 h before the addition of allogeneic T cells (2 × 105). DC – dendritic cells cultured without allogeneic T cells; TC-allogeneic T cells cultured without dendritic cells. Data are mean ± SEM of 3 experiments. Proliferation was assessed by incorporation of 3H-thymidine.


References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

