Respiratory syncytial virus and neutrophil activation
- PMID: 15932508
- PMCID: PMC1809401
- DOI: 10.1111/j.1365-2249.2005.02780.x
Respiratory syncytial virus and neutrophil activation
Abstract
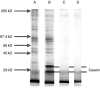
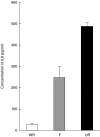
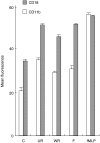
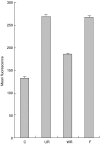
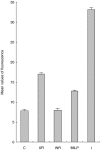
Respiratory syncytial virus infects almost all children by 2 years of age. Neutrophils are the predominant airway leucocytes in RSV bronchiolitis and they are activated in the presence of infection. However it is not clear whether RSV can directly signal to activate neutrophil cytotoxic function. To investigate this we have used a preparation of RSV washed using a new centrifugal diafiltration method to rapidly remove inflammatory molecules produced by the epithelial cells used to propagate the RSV stock. Human neutrophils were isolated from peripheral blood and activated with either the unwashed crude RSV preparations or the purified intact RSV. Neutrophils were also challenged with purified RSV G-glycoprotein. The effect of challenging human neutrophils with these preparations of intact RSV, or the RSV G-glycoprotein, was assessed by measuring the cell surface expression of CD11b and CD18b, the phagocytic oxidative burst, and intracellular release of calcium pools. Neutrophils challenged with the washed RSV exhibited significantly lower activation of surface marker expression (P < 0.001) and oxidative burst (P < 0.001) than those challenged with unwashed virus or with virus free supernatant. There was no increase in intracellular calcium release on exposure to the washed RSV. Purified G glycoprotein did not stimulate neutrophils, whilst the use of a blocking antibody to the F protein did not prevent unwashed RSV from activating cytotoxic responses. These results suggest that neutrophils have no innate signalling system that recognizes RSV but they are activated at sites of RSV infection as a result of the cytokines and inflammatory molecules released by virally infected cells.
Figures
References
-
- Everard ML. Respiratory syncytial virus bronchiolitis and pneumonia. In: Taussig L, Landau L, editors. Textbook of Paediatric Respiratory Medicine. St Louis: Mosby; 1998. pp. 580–94.
-
- Gilchrist S, Torok TJ, Gary HE, Jr, Alexander JP, Anderson LJ. National surveillance for respiratory syncytial virus, United States, 1985–90. J Inf Dis. 1994;170:986–90. - PubMed
-
- Fleming DM, Cross KW. Respiratory syncytial virus or influenza. Lancet. 1993;342:1507–10. - PubMed
-
- Smith P, Wang SZ, Dowling K, Forsyth K. Leukocyte populations in respiratory syncytial virus-induced bronchiolitis. J Paediatr Child Health. 2001;37:146–51. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

