Ca2+ permeability of nicotinic acetylcholine receptors in rat hippocampal CA1 interneurones
- PMID: 15932886
- PMCID: PMC1464780
- DOI: 10.1113/jphysiol.2005.089789
Ca2+ permeability of nicotinic acetylcholine receptors in rat hippocampal CA1 interneurones
Erratum in
- J Physiol. 2005 Nov 1;568(Pt 3):1067
Abstract
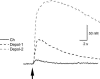
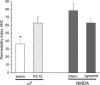
Neuronal nicotinic acetylcholine receptors (nAChRs) are widely expressed in the brain where they are involved in a variety of physiological processes, including cognition and development. The nAChRs are ligand-gated cationic channels, and different subtypes are known to be differentially permeable to Ca2+; the alpha7-containing nAChRs are generally considered to be the most permeable. Ca2+ can activate and regulate a variety of signal transduction cascades, and the influx of Ca2+ through these receptors may have implications for synaptic plasticity. To determine the Ca2+ permeability of the nAChRs in rat hippocampal interneurones in the slice, which contain diverse subtypes of alpha7- and non-alpha7-containing nAChRs, we combined patch-clamp electrophysiology recordings with conventional fura-2 fluorescence imaging techniques. We estimated the relative Ca2+ permeability of the channels by determining the ratio of the increase in [Ca2+]i level (Delta[Ca2+]i) in the soma to the integrated transmembrane current (charge, Q) induced by the activation of the nAChRs, and compared this ratio to the highly Ca2+ permeable NMDA subtype of glutamate receptor channel. In all cells tested, the Delta[Ca2+]i/Q ratio was significantly larger (i.e. more than twice as big) for responses activated by NMDA than for alpha7-containing nAChRs in interneurones; the activation of the non-alpha7 nAChRs did not produce any significant increase in [Ca2+]i. Interestingly, the Ca2+ permeability of native alpha7 nAChRs in PC12 cells was significantly larger than in hippocampal interneurones, and not significantly different from NMDA receptors. Therefore, the alpha7-containing nAChRs in rat hippocampal interneurones are significantly less permeable to Ca2+ than not only NMDA receptors but also alpha7 nAChRs in PC12 cells.
Figures


Similar articles
-
Dendritic Ca2+ signalling due to activation of alpha 7-containing nicotinic acetylcholine receptors in rat hippocampal neurons.J Physiol. 2007 Jul 15;582(Pt 2):597-611. doi: 10.1113/jphysiol.2007.135319. Epub 2007 May 17. J Physiol. 2007. PMID: 17510177 Free PMC article.
-
Rat nicotinic acetylcholine receptor alpha2beta2 channels: comparison of functional properties with alpha4beta2 channels in Xenopus oocytes.Neuroscience. 2004;124(4):817-22. doi: 10.1016/j.neuroscience.2004.01.017. Neuroscience. 2004. PMID: 15026122
-
Paired-pulse potentiation of alpha7-containing nAChRs in rat hippocampal CA1 stratum radiatum interneurones.J Physiol. 2005 Nov 1;568(Pt 3):881-9. doi: 10.1113/jphysiol.2005.096081. Epub 2005 Sep 1. J Physiol. 2005. PMID: 16141265 Free PMC article.
-
Nicotinic acetylcholine receptors of adrenal chromaffin cells.Acta Physiol (Oxf). 2008 Feb;192(2):203-12. doi: 10.1111/j.1748-1716.2007.01804.x. Epub 2007 Nov 15. Acta Physiol (Oxf). 2008. PMID: 18005395 Review.
-
The alpha7 nicotinic acetylcholine receptor in neuronal plasticity.Mol Neurobiol. 1999 Aug;20(1):1-16. doi: 10.1007/BF02741361. Mol Neurobiol. 1999. PMID: 10595869 Review.
Cited by
-
Cholinergic receptors: functional role of nicotinic ACh receptors in brain circuits and disease.Pflugers Arch. 2013 Apr;465(4):441-50. doi: 10.1007/s00424-012-1200-1. Epub 2013 Jan 11. Pflugers Arch. 2013. PMID: 23307081 Free PMC article. Review.
-
Differential signalling induced by α7 nicotinic acetylcholine receptors in hippocampal dentate gyrus in vitro and in vivo.J Physiol. 2021 Oct;599(20):4687-4704. doi: 10.1113/JP280505. Epub 2021 Sep 28. J Physiol. 2021. PMID: 34487349 Free PMC article.
-
Nicotine facilitates long-term potentiation induction in oriens-lacunosum moleculare cells via Ca2+ entry through non-alpha7 nicotinic acetylcholine receptors.Eur J Neurosci. 2010 Feb;31(3):463-76. doi: 10.1111/j.1460-9568.2009.07058.x. Epub 2010 Jan 26. Eur J Neurosci. 2010. PMID: 20113344 Free PMC article.
-
Acetylcholine and antibodies against the acetylcholine receptor protect neurons and astrocytes against beta-amyloid toxicity.Int J Biochem Cell Biol. 2013 Apr;45(4):899-907. doi: 10.1016/j.biocel.2013.01.011. Epub 2013 Jan 23. Int J Biochem Cell Biol. 2013. PMID: 23353645 Free PMC article.
-
Nicotine elicits prolonged calcium signaling along ventral hippocampal axons.PLoS One. 2013 Dec 5;8(12):e82719. doi: 10.1371/journal.pone.0082719. eCollection 2013. PLoS One. 2013. PMID: 24349346 Free PMC article.
References
-
- Anand R, Peng X, Lindstrom J. Homomeric and native α7 acetylcholine receptors exhibit remarkably similar but non-identical pharmacological properties, suggesting that the native receptor is a heteromeric protein complex. FEBS Lett. 1993;327:241–246. - PubMed
-
- Berg DK, Conroy WG. Nicotinic α7 receptors: synaptic options and downstream signaling in neurons. J Neurobiol. 2002;53:512–523. - PubMed
-
- Berridge MJ. Inositol trisphosphate and calcium signalling. Nature. 1993;361:315–325. - PubMed
-
- Berridge MJ, Bootman MD, Roderick HL. Calcium signalling: dynamics, homeostasis and remodeling. Nat Rev Mol Cell Biol. 2003;4:517–529. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

