Midline serotonergic neurones contribute to widespread synchronized activity in embryonic mouse hindbrain
- PMID: 15932887
- PMCID: PMC1464778
- DOI: 10.1113/jphysiol.2005.089581
Midline serotonergic neurones contribute to widespread synchronized activity in embryonic mouse hindbrain
Abstract
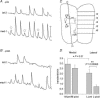
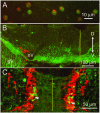
Spontaneous, synchronous activity occurs in motor neurones of the embryonic mouse hindbrain at the stage when rhombomeric segmentation disappears (embryonic day 11.5). The mechanisms generating and synchronizing the activity, however, and the extent to which it is widespread in the hindbrain, are unknown. We show here that spontaneous activity is initiated in the midline of the hindbrain, and propagates laterally to encompass virtually the entire hindbrain synchronously and bilaterally. Separation of the midline region from lateral regions abolishes or slows activity laterally, but not medially. The early differentiating neurones of the midline raphe system are present in the rostral midline and express serotonin at E11.5. Their axons ramify extensively in the marginal zone, cross the midline, and extend at the midline both rostrally into the midbrain and caudally towards the caudal hindbrain. Blockers of serotonin receptors, specifically the 5-HT(2A) receptor, abolish synchronous activity in the hindbrain, while blockers of other neurotransmitter systems, including GABA and glutamate, do not. In addition, the 5-HT(2A) receptor is expressed in the marginal regions in the entire medial-to-lateral extent of the hindbrain and in the midline commissural region. Thus, the serotonergic neurones of the developing midline raphe system may play a role in initiating and propagating spontaneous synchronous activity throughout the hindbrain.
Figures






Comment in
-
Serotonergic neurones drive spontaneous activity in the developing mouse hindbrain.J Physiol. 2005 Aug 1;566(Pt 3):643. doi: 10.1113/jphysiol.2005.093278. Epub 2005 Jun 30. J Physiol. 2005. PMID: 15994178 Free PMC article. No abstract available.
-
Problems of drug selectivity and dose--pharmacology.J Physiol. 2005 Dec 1;569(Pt 2):711; author reply 712. doi: 10.1113/jphysiol.2005.569007. J Physiol. 2005. PMID: 16322062 Free PMC article. No abstract available.
Similar articles
-
Serotonergic neurones drive spontaneous activity in the developing mouse hindbrain.J Physiol. 2005 Aug 1;566(Pt 3):643. doi: 10.1113/jphysiol.2005.093278. Epub 2005 Jun 30. J Physiol. 2005. PMID: 15994178 Free PMC article. No abstract available.
-
Properties and mechanisms of spontaneous activity in the embryonic chick hindbrain.Dev Neurobiol. 2009 Jul;69(8):477-90. doi: 10.1002/dneu.20712. Dev Neurobiol. 2009. PMID: 19263418
-
Primary role of the serotonergic midline system in synchronized spontaneous activity during development of the embryonic mouse hindbrain.J Neurobiol. 2006 Sep 15;66(11):1239-52. doi: 10.1002/neu.20259. J Neurobiol. 2006. PMID: 16902991
-
The role of dorsal raphe nucleus serotonergic and non-serotonergic neurons, and of their receptors, in regulating waking and rapid eye movement (REM) sleep.Sleep Med Rev. 2010 Oct;14(5):319-27. doi: 10.1016/j.smrv.2009.10.003. Epub 2010 Feb 12. Sleep Med Rev. 2010. PMID: 20153670 Review.
-
The structure of the dorsal raphe nucleus and its relevance to the regulation of sleep and wakefulness.Sleep Med Rev. 2010 Oct;14(5):307-17. doi: 10.1016/j.smrv.2009.11.004. Epub 2010 Feb 12. Sleep Med Rev. 2010. PMID: 20153669 Review.
Cited by
-
Developmental changes in propagation patterns and transmitter dependence of waves of spontaneous activity in the mouse cerebral cortex.J Physiol. 2011 May 15;589(Pt 10):2529-41. doi: 10.1113/jphysiol.2010.202382. Epub 2011 Mar 28. J Physiol. 2011. PMID: 21486817 Free PMC article.
-
Serotonergic neurones drive spontaneous activity in the developing mouse hindbrain.J Physiol. 2005 Aug 1;566(Pt 3):643. doi: 10.1113/jphysiol.2005.093278. Epub 2005 Jun 30. J Physiol. 2005. PMID: 15994178 Free PMC article. No abstract available.
-
Regulation of Spontaneous Propagating Waves in the Embryonic Mouse Brainstem.Front Neural Circuits. 2017 Jan 4;10:110. doi: 10.3389/fncir.2016.00110. eCollection 2016. Front Neural Circuits. 2017. PMID: 28101007 Free PMC article.
-
The role of serotonin in respiratory function and dysfunction.Respir Physiol Neurobiol. 2010 Nov 30;174(1-2):76-88. doi: 10.1016/j.resp.2010.08.017. Epub 2010 Aug 27. Respir Physiol Neurobiol. 2010. PMID: 20801236 Free PMC article. Review.
-
Large-scale synchronized activity in the embryonic brainstem and spinal cord.Front Cell Neurosci. 2013 Apr 5;7:36. doi: 10.3389/fncel.2013.00036. eCollection 2013. Front Cell Neurosci. 2013. PMID: 23596392 Free PMC article.
References
-
- Barnes NM, Sharp T. A review of central 5-HT receptors and their function. Neuropharmacology. 1999;38:1083–1152. - PubMed
-
- Ben-Ari Y. Excitatory actions of GABA during development: the nature of the nurture. Nature Rev Neurosci. 2002;3:728–739. - PubMed
-
- Bruning G, Liangos O, Baumgarten HG. Prenatal development of the serotonin transporter in mouse brain. Cell Tissue Res. 1997;289:211–221. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

